CCTS supports every stage of the clinical trial lifecycle. Just click any box to learn more.
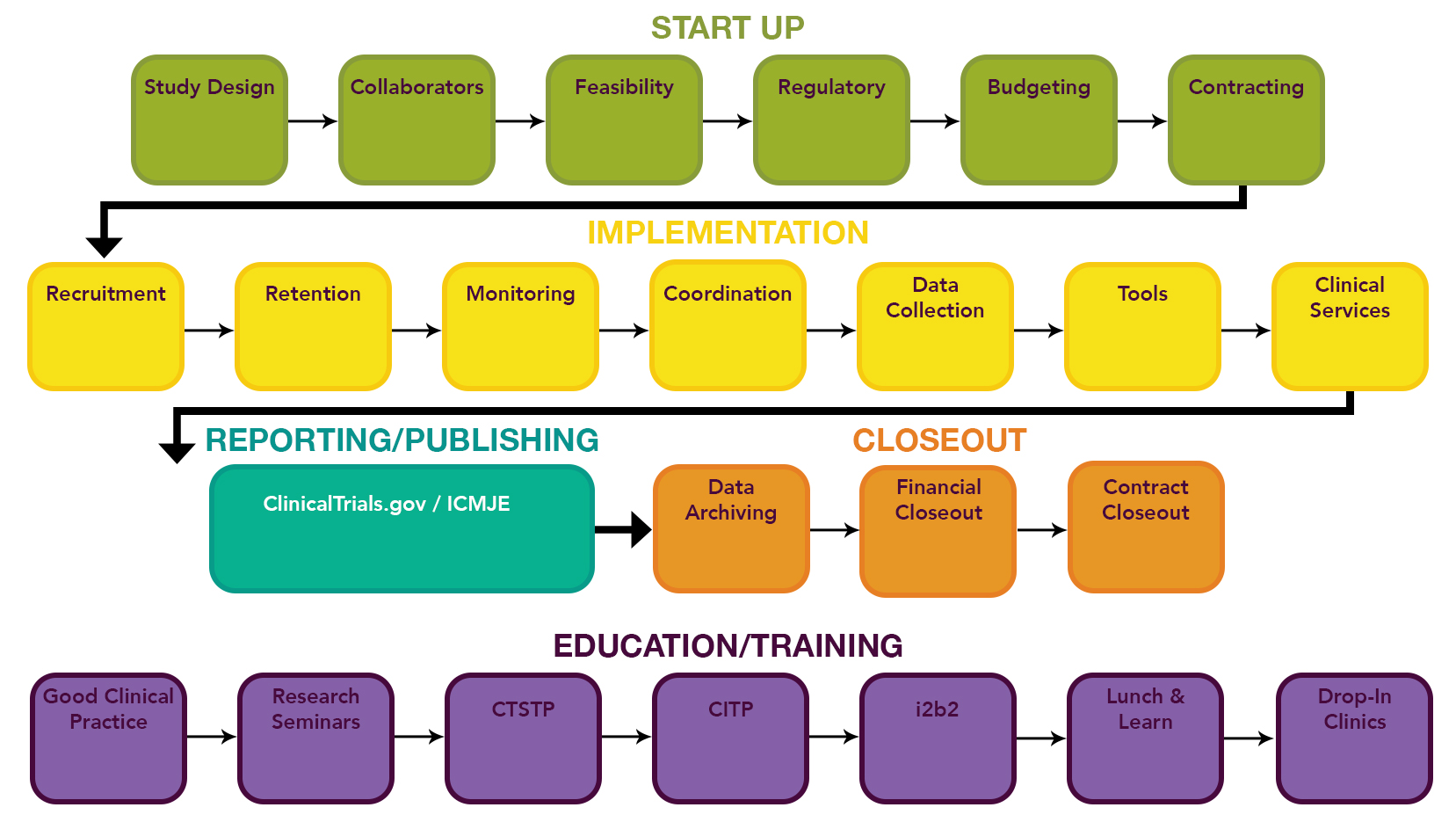
Study Design
Start with CCTS panels and the BERD to help strengthen your study design. CRSP can review and provide feedback on the implementation of your study design. your clinical study’s protocol. Learn more
Collaborators
Contact Research Commons to find collaborators via our Informatics Gateway, TIES panels, Partner Network, and other team science platforms. Learn more
Feasibility
CRSP will help you assess the feasibility of your trial. Our BERD unit can guide you through a power analysis. CCTS Informatics supports investigator access to clinical data. Check out our feasibility tool.
Regulatory
CRSP is your one-stop shop for help with IRB, IND/IDE forms, ClinicalTrials.gov requirements, & FDA and internal audits. For IND questions, please contact This email address is being protected from spambots. You need JavaScript enabled to view it. . Learn more
Budgeting
CRSP staff are experts in building budgets for clinical trials. Watch Budget Best Practices Video.
Contracting
OSP can help you negotiate better terms for your trial contracts. Learn more
Recruitment
CCTS offers several recruitment supports: our i2b2 database empowers researchers to search aggregate or limited clinical data for potential cohorts; our CRSP unit can help develop and seek IRB approval for recruitment plans as well as help identify, pre-screen, enroll, and consent participants. Learn more
Retention
Our trained, experienced, and certified CRSP coordinators can help you keep the participants you enroll. Learn more
Monitoring
Our trained, experienced, and certified CRSP coordinators provide monitoring support. Learn more
Coordination
Our trained, experienced, and certified CRSP coordinators will help keep your trial moving smoothly. Learn more
Data Collection
Our trained, experienced, and certified CRSP staff will help collect and enter your study’s data. Learn more
Tools
CCTS offers a library of helpful implementation tools for clinical trials. Visit the Clinical Trials Kiosk
Clinical Services
CCTS offers bionutritionists, certified research nurses and trial coordinators, two children’s research environments, a Phase I clinical trials unit, specimen processing and analysis, a biorepository, OnCore specialists, and more. Consultations available! Learn more
ClinicalTrials.gov / ICMJE
CRSP can guide you through reporting your data in ClinicalTrials.gov and update you on the latest requirements for publishing by the International Committee of Medical Journal Editors (ICMJE). Learn more
Data Archiving
CRSP can help your team with labor intensive close out steps, like archiving data. Learn more
Financial Closeout
CRSP can help your team with labor intensive close out steps, like invoicing final billables. Learn more
Good Clinical Practice
Our CRSP unit ensures all CCTS clinical trainings reinforce Good Clinical Practice (GCP) standards of excellence. CRSP also offers GCP consults. Learn more
Research Seminars
Twice a month trainings fill in knowledge gaps identified by research teams. Learn more
CTSTP
Our Clinical Translational Science Training Program (CTSTP) is a six-month certification program offering ~50 hours of didactic and interactive in key research competencies. Learn more
CITP
Our Clinical Investigator Training Program (CITP) is held in the Spring and Fall and tailored to new clinical investigators and trialists, raising awareness of Good Clinical Practice standards and research capacities and expertise to support safety and rigor in trials. Learn more
i2b2
This one-time, two-hour hands-on training will empower you to access clinical data for research. Learn more
Lunch & Learn
Quarterly guidance on the latest changes in clinical trial policies and resources. Invaluable! Learn more
Drop-In Clinics
Bring your burning questions (and a laptop) to a CCTS clinic—BERD experts are available twice a week; IRB, Biorepository, Bionutrition, and CRU clinics happen monthly. Learn more
-
Study Design
Start with CCTS panels and the BERD to help strengthen your study design. CRSP can review and provide feedback on the implementation of your study design. your clinical study’s protocol. Learn more
-
Collaborators
Contact Research Commons to find collaborators via our Informatics Gateway, TIES panels, Partner Network, and other team science platforms. Learn more
-
Feasibility
CRSP will help you assess the feasibility of your trial. Our BERD unit can guide you through a power analysis. CCTS Informatics supports investigator access to clinical data. Check out our feasibility tool.
-
Regulatory
CRSP is your one-stop shop for help with IRB, IND/IDE forms, ClinicalTrials.gov requirements, & FDA and internal audits. For IND questions, please contact
This email address is being protected from spambots. You need JavaScript enabled to view it. . Learn more -
Budgeting
CRSP staff are experts in building budgets for clinical trials. Watch Budget Best Practices Video.
-
Contracting
OSP can help you negotiate better terms for your trial contracts. Learn more
-
Recruitment
CCTS offers several recruitment supports: our i2b2 database empowers researchers to search aggregate or limited clinical data for potential cohorts; our CRSP unit can help develop and seek IRB approval for recruitment plans as well as help identify, pre-screen, enroll, and consent participants. Learn more
-
Retention
Our trained, experienced, and certified CRSP coordinators can help you keep the participants you enroll. Learn more
-
Monitoring
Our trained, experienced, and certified CRSP coordinators provide monitoring support. Learn more
-
Coordination
Our trained, experienced, and certified CRSP coordinators will help keep your trial moving smoothly. Learn more
-
Data Collection
Our trained, experienced, and certified CRSP staff will help collect and enter your study’s data. Learn more
-
Tools
CCTS offers a library of helpful implementation tools for clinical trials. Visit the Clinical Trials Kiosk
-
Clinical Services
CCTS offers bionutritionists, certified research nurses and trial coordinators, two children’s research environments, a Phase I clinical trials unit, specimen processing and analysis, a biorepository, OnCore specialists, and more. Consultations available! Learn more
-
ClinicalTrials.gov / ICMJE
CRSP can guide you through reporting your data in ClinicalTrials.gov and update you on the latest requirements for publishing by the International Committee of Medical Journal Editors (ICMJE). Learn more
-
Data Archiving
CRSP can help your team with labor intensive close out steps, like archiving data. Learn more
-
Financial Closeout
CRSP can help your team with labor intensive close out steps, like invoicing final billables. Learn more
-
Contract Closeout
CRSP can provide guidance on closeout.
-
Good Clinical Practice
Our CRSP unit ensures all CCTS clinical trainings reinforce Good Clinical Practice (GCP) standards of excellence. CRSP also offers GCP consults. Learn more
-
Research Seminars
Twice a month trainings fill in knowledge gaps identified by research teams. Learn more
-
CTSTP
Our Clinical Translational Science Training Program (CTSTP) is a six-month certification program offering ~50 hours of didactic and interactive in key research competencies. Learn more
-
CITP
Our Clinical Investigator Training Program (CITP) is held in the Spring and Fall and tailored to new clinical investigators and trialists, raising awareness of Good Clinical Practice standards and research capacities and expertise to support safety and rigor in trials. Learn more
-
i2b2
This one-time, two-hour hands-on training will empower you to access clinical data for research. Learn more
-
Lunch & Learn
Quarterly guidance on the latest changes in clinical trial policies and resources. Invaluable! Learn more
-
Drop-In Clinics
Bring your burning questions (and a laptop) to a CCTS clinic—BERD experts are available twice a week; IRB, Biorepository, Bionutrition, and CRU clinics happen monthly. Learn more
