The Bio-Analytical Redox Biology (BARB) Core provides state-of-the-art services in mitochondrial metabolism, oxidative stress, psychological and physiological stress assessment research in diabetes, obesity, exercise, pediatric neonatology, cancer, aging, optometry, microbiology, neurobiology, nutrition, pain, psychology, urology, and cardiometabolic disease research on a fee-for-service basis. The BARB Core has provided services to over 350 UAB and external principal investigators (PIs) since its inception in 2008 and provides a combination of services not available through any other UAB cores or regional universities. The BARB Core serves as a hub to facilitate and promote the development of multi-disciplinary research projects among independently funded research programs at UAB, nationally and worldwide.
The BARB Core is a Diabetes Research Center Metabolic and Redox Sub Core (NIDDK P30DK079626) as well as a UAB Institutional Research Pilot Core. We are also supported by several UAB Centers: Nutrition Obesity Research Center (NIDDK P30DK056336), Center for Clinical and Translational Science (NIH UL1TR003096), Center for Free Radical Biology and UAB Center for Exercise Medicine.
Objectives
- To provide services in mitochondrial bioanalytical and damage analyses and oxidative stress assessment using state-of-the-art research facilities, specialized expertise, and quality control.
- To provide access to centralized resources and our expert staff, substantially reducing equipment, personnel labor costs, and investigator time commitment.
- To provide opportunities to learn, train and develop new research methodologies through initial consultations, workshops and one-on-one on training to enable optimal usage and growth of the various services of this core.
- To keep our investigators apprised of current advances in bioenergetics, metabolism, and oxidant stress quantification through informative emails, our website and social media, to ensure we remain at the forefront of ‘gold-standard’ methodologies.
- To continue to develop, design and optimize cutting-edge, pioneering techniques and protocols that allow the investigator to extend capabilities, precision, and foster multidisciplinary collaborations.
- To provide a unique portfolio of services that does not duplicate any other UAB core facility to strengthen and align with UAB’s Research Mission “to contribute new knowledge and growth of the economies of the city and state.”
- To use feedback to continue to maintain high standards of quality, improve and increase services offered, as well as adapt to the changing needs of the user base.
Service Request Form
We are happy to assist you and look forward to working with you. To schedule a free initial 15-minute Zoom consultation, request a letter of support, inquire about a service/training or any other request, please use this link or scan the QR code: https://forms.office.com/r/6mDGk2XYuY

Services
All BARB Core Assays are of the highest quality, rigorously optimized, reproducible and standardized. Training is available for High Resolution Respirometry, Seahorse XFe96 Bioenergetics and Mitochondrial Electron Transport Chain (ETC) Kinetics. Please contact us about any other desired training.
Assays
Hormonal chronic stress/cortisol analyses
Sample: human hair, finger/toenails (human)
Non-invasive and can be collected, stored and shipped to the BARB Core at room temperature, using regular mail, without any special biohazard requirements or costs.
Equipment: Qiagen TissueLyser III, Biotek Synergy 2 Multi-mode microplate reader
Publications: dietary inflammation, discrimination and stress, chronic cortisol in adult cancer survivors, gardening intervention trial in cancer survivors.
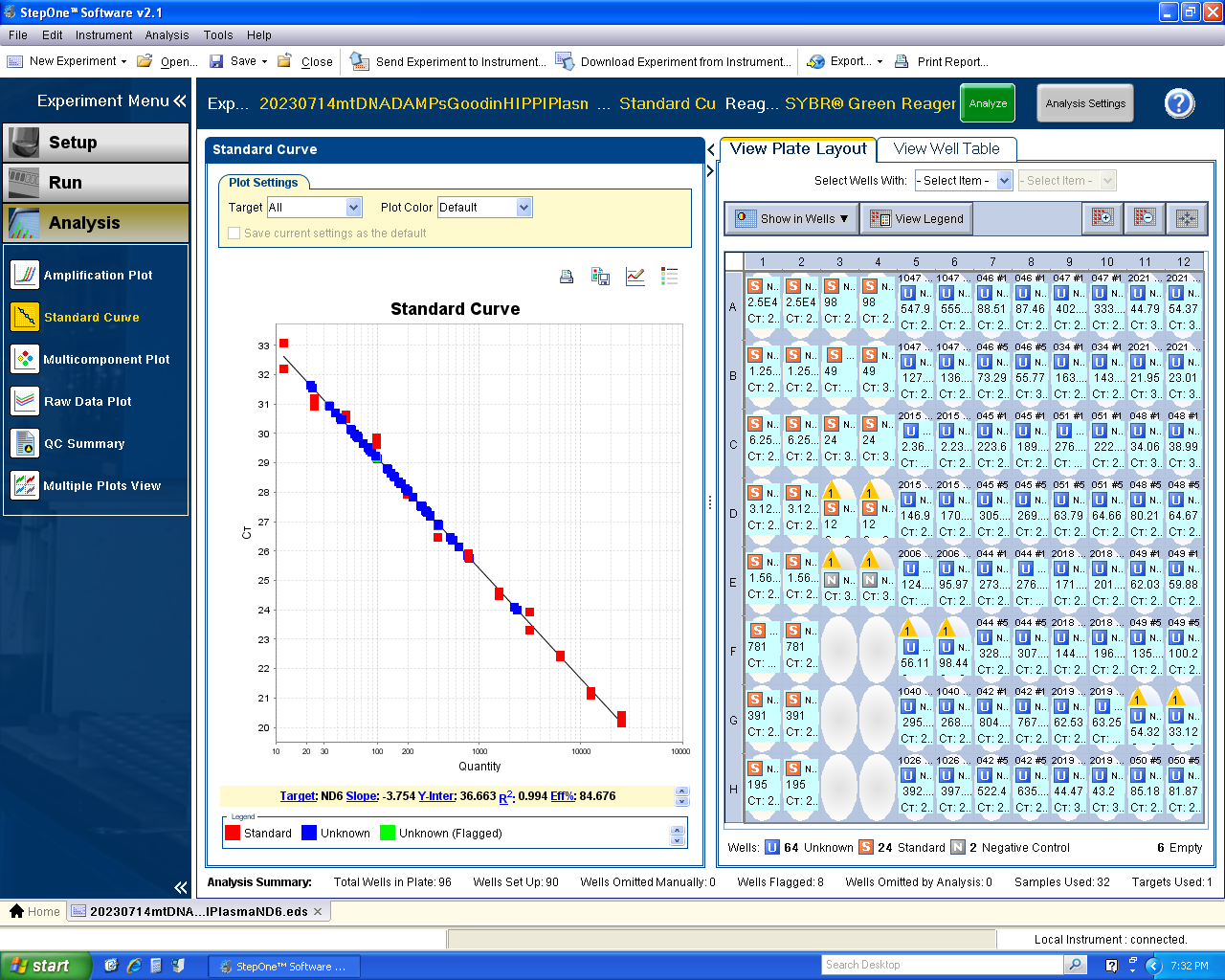
 Mitochondrial DNA Damage-Associated Molecular Patterns (mtDNA DAMPs)
Mitochondrial DNA Damage-Associated Molecular Patterns (mtDNA DAMPs)
Sample: frozen serum/plasma (human, mouse)
Equipment: Applied Biosystems StepOnePlus Real-Time PCR System, Applied Biosystems DynaMag Magnet, Biotek Synergy 2 Multi-Mode Microplate Reader, LabNet Plate Spinner
Publications: HIV and Chronic Pain
Mitochondrial Electron Transport Chain (ETC) Kinetics:
- Complex I (NADH:Ubiquinone Oxidoreductase) Assay
- Complex II (Succinate-Ubiquinone Oxidoreductase) Assay
- Complex III (Decylubiquinol Cytochrome C oxidoreductase) Assay
- Complex IV (Cytochrome c oxidase) Assay
- Complex V (ATP hydrolysis) Assay
- Citrate Synthase Assay
Sample: fresh or frozen tissues or cells (human, rodent, fish, Drosophila)
Equipment: Beckman DU 800 Spectrophotometer, Custom-Built Mitochondrial Isolation Stations with Recirculating Chiller
Publications: skeletal muscle in rheumatoid arthritis, TrxR2 and glucose tolerance, neonatal hippocampus development, brain IGF-1 and aging, mitochondrial fusion and exercise
Mitochondrial Bioenergetics:
- High Resolution Respirometry (HRR) - Oxidative Phosphorylation ONLY
Sample: fresh, permeabilized, ex vivo tissues (human muscle, rodent renal cortex, medulla, and microvessels, embryonic and adult heart, gastroc and soleus muscle, hippocampus, bladder, liver) or isolated mitochondria (rodent, fish, Drosophila)
Equipment: 3 x Oroboros Oxygraph-2k Fluorometers (LED2-Module Amperometric Add-On)
Publications: neonatal lung injury, skeletal muscle in rheumatoid arthritis, embryonic heart cardiogenesis, soleus muscle in spinal cord injury, post-exercise skeletal muscle, neonatal hippocampus development, aerobic exercise training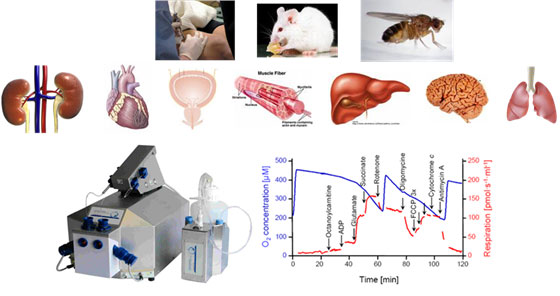
- Seahorse Extracellular Flux Analysis (XFe96) – Oxidative Phosphorylation and Glycolysis
Sample: cultured cells or freshly isolated mitochondria (human, rodent), mycoplasmas.
Equipment: Agilent Seahorse XFe96, high throughput, 96-well format
Publications: neonatal lung injury, ovarian cancer, breast cancer, bronchopulmonary dysplasia, adrenal neuroendocrine cancer, glucose tolerance, mammary tumor-associated macrophages, diabetic retinopathy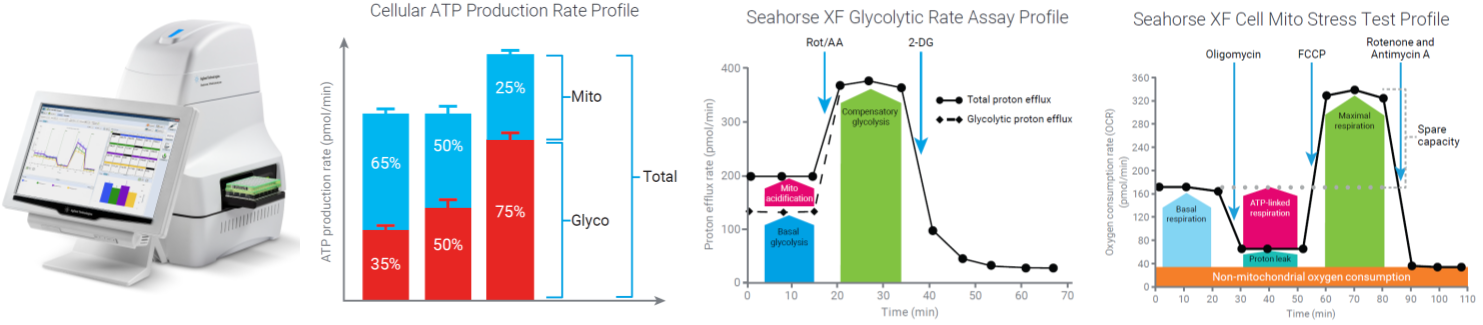
Mitochondrial Isolation: Extremely high-quality, reproducible, standardized.
- For High Resolution Respirometry (HRR)
- For Mitochondrial Electron Transport Chain (ETC) Kinetics
Redox Assays:
- Catalase
Sample: plasma, serum, total homogenate - Glutathione Tietze (GSSG and GSH)
Sample: plasma, whole blood - Glutathione Peroxidase (GPX)
Sample: plasma, total homogenate - Total Antioxidant Capacity (TAC)
Sample: plasma, serum, urine, saliva - Ferric Reducing Antioxidant Power (FRAP)
Sample: plasma
If there are other assays of interest that we currently do not offer, or to schedule your free consult please contact us.
BARB Core Lab Clean Up & Close Out Services
- To request our lab clean up or close out services please fill out the service request form using this link: https://forms.office.com/r/6mDGk2XYuY
- Once we receive the request, we will schedule a free 15-min Zoom consultation where we can discuss and coordinate the services requested.
- Then we will contact EH&S on your behalf and notify them of the intent to move/closeout these lab spaces, tag out equipment, and schedule a tour of the lab spaces with an EH&S Research Safety Representative to provide guidance on proper procedures to be followed.
- Then we will tour the spaces together and our team can begin the clean out.
We are fully certified by UAB EH&S to do:
- biohazard waste removal
- chemical segregating/packing/manifesting
- equipment inventorying, decontaminating, and tagging for surplus (usually offered up to other PIs in the department first along with consumables)
- cleaning and boxing up glassware for donation to Green Labs (offered to department PIs first)
- closing out labs
Our services are first come first served. We would prefer as much notice as you can give us but if you have a deadline or special circumstances, we will do our best to expedite the process. We typically have 3 team members that work on these cleanouts. Our fees are $100/h per team member, if all 3 of us work at once we get it done 3x as fast, but the total cost remains the same as if one of us worked and it takes 3x as long. This allows us to be flexible and get the clean ups/clean outs done as efficiently as possible.
See what the UAB community has to say about working with the BARB Core Team to clean up and close out labs across campus: https://www.uab.edu/reporter/in-the-know/uab-barb-core-handles-the-tough-tasks-of-cleaning-out-labs
Training
Training is available for most assays and associated equipment, our most popular being: High Resolution Respirometry, Seahorse XFe96 Bioenergetics and Mitochondrial Electron Transport Chain (ETC) Kinetics.
If you would like to be trained in any of these or other assays and/or to schedule your free consult, please contact us.
Equipment
- Agilent Seahorse Extracellular Flux Analyzer XFe96
- 3x Oroboros Oxygraph-2k High Resolution Respirometry Systems
- Fluorescence LED2-Module Amperometric Add-On for Oroboros Oxygraph-2k
- Qiagen TissueLyser III
- Biotek Synergy 2 Multi-Mode Microplate Reader (fluorescence, luminescence, absorbance)
- Beckman DU 800 Spectrophotometer
- Zeiss Stemi 2000 Stereomicroscope
- Applied Biosystems StepOnePlus Real-Time PCR System
- Applied Biosystems DynaMag Magnet
- Single and Dual Custom-Built Mitochondrial Isolation Stations with Recirculating Chiller
- Parr Cell Disruption by Nitrogen Decompression
- Krumdieck Tissue Slicer (Alabama Research and Development) with Coring Press, Embedding Unit, Incubation Unit, Thickness Gauge, Recirculating Chiller
- LabNet Plate Spinner
- Biosafety Cabinet for Tissue Culture
- Stacked Incubators (2) for Tissue Culture
Please contact us if you would like to be trained to use any of this equipment and/or to schedule your free consult.
Reservations
If you have already received training from the experts at the BARB Core, and would like to reserve a piece of equipment, you may do so using the FBS Calendar. Please email mjsammy@uab.edu and johnk@uab.edu or call us at (205) 996-2661 if you have any issues.
Tell us how we are doing
Please let us know what we did well, in what areas we can improve, and what services you would like us to offer. Your feedback is greatly appreciated and helps us to better serve you and the UAB community. Satisfaction Survey: https://forms.office.com/r/D6JSpXSiN5
Acknowledgement
Please acknowledge our BARB Core on all presentations and publications and share your publication citations with us once they are accepted. This helps to ensure our ability to continue to provide services to the UAB research community.
Acknowledgement: Melissa J. Sammy, PhD and Kelley E. Smith-Johnston, BS at the UAB Bio-Analytical Redox Biology (BARB) Core, DRC (NIDDK P30DK079626), NORC (NIDDK P30DK056336), CCTS (NIH UL1TR003096), CFRB and UCEM
Send us your Accomplishments
 We want to celebrate your achievements! Email citations for your publications, abstracts, presentations, or awarded grants utilizing BARB Core services to us at mjsammy@uab.edu or johnk@uab.edu. We will proudly feature your accomplishments in our internal reports, website, social media and cite your publications on methods sections provided to future BARB Core patrons.
We want to celebrate your achievements! Email citations for your publications, abstracts, presentations, or awarded grants utilizing BARB Core services to us at mjsammy@uab.edu or johnk@uab.edu. We will proudly feature your accomplishments in our internal reports, website, social media and cite your publications on methods sections provided to future BARB Core patrons.
Social Media
Check us out on social media and don’t forget to email us your accomplishments so we can proudly feature your successes! Check back soon for links to our social media accounts.
We are Committed to Sustainability
 The BARB Core is Green Lab Certified at the bronze level. This nationally recognized certification demonstrates that our lab has significantly reduced energy, water, waste, and hazardous chemical use, leading to a safer, more sustainable work environment. We conserve, share, and reuse resources whenever possible and we are mindful of the impact of our work. Our lab operates as efficiently as possible, and we work closely with the Office of Sustainability, EH&S, and Facilities Management to ensure that it continues to do so into the future. Our efforts in reducing our resources and sharing equipment contribute to avoided costs for our university and federal grants. As a bronze-certified lab, we promote stewardship of taxpayer support and reduce the environmental impacts of NIH-supported research projects.
The BARB Core is Green Lab Certified at the bronze level. This nationally recognized certification demonstrates that our lab has significantly reduced energy, water, waste, and hazardous chemical use, leading to a safer, more sustainable work environment. We conserve, share, and reuse resources whenever possible and we are mindful of the impact of our work. Our lab operates as efficiently as possible, and we work closely with the Office of Sustainability, EH&S, and Facilities Management to ensure that it continues to do so into the future. Our efforts in reducing our resources and sharing equipment contribute to avoided costs for our university and federal grants. As a bronze-certified lab, we promote stewardship of taxpayer support and reduce the environmental impacts of NIH-supported research projects.
Contact Us
Our BARB Core Team members are happy to assist you and look forward to working with you. To schedule a free initial 15-minute Zoom consultation, request a letter of support, inquire about a service/training or any other request, please use this link: https://forms.office.com/r/6mDGk2XYuY

Director: Doug Moellering, Ph.D.
WEBB 429
1675 University Blvd.
Phone: (205) 996-2660
Fax: (205) 996-5896
Email: dmoellering@uab.edu

Operational Director: Melissa Sammy, Ph.D.
WEBB 416 & 418
1675 University Blvd.
Phone: (205) 996-2661
Fax: (205) 996-5896
Email: mjsammy@uab.edu

Research Assistant: Kelley Johnston
WEBB 418
1675 University Blvd.
Phone: (205) 996-2661
Fax: (205) 996-5896
Email: johnk@uab.edu
Publications
2023 [1-6]
- Vamesu, B.M., et al. Thyroid Hormone modulates hyperoxic neonatal lung injury and mitochondrial function. JCI Insight. 2023. 8(8):e160697
- Moellering, D.R., et al. Association between skeletal muscle mitochondrial dysfunction and insulin resistance in patients with rheumatoid arthritis: a case-control study. Arthritis Res Ther. 2023. 25(1):85.
- Kandasamy, J., et al. Mitochondrial DNA Variations Modulate Alveolar Epithelial Mitochondrial Function and Oxidative Stress in Newborn Mice Exposed to Hyperoxia. bioRxiv [Preprint]. 2023. 17:2023.05.17.541177.
- Jones, R.B., et al. Role of the ST6GAL1 sialyltransferase in regulating ovarian cancer cell metabolism. Glycobiology. 2023. cwad051.
- Hinshaw, D.C.,. Hedgehog Signaling Regulates Treg to Th17 Conversion Through Metabolic Rewiring in Breast Cancer. Cancer Immunol Res. 2023. 11(5):687-702.
- Gilstrap, S.R., et al., Mitochondrial Reactivity Following Acute Exposure to Experimental Pain Testing in People with HIV and Chronic Pain. Mol Pain. 2023.
2022 [7-14]
- Zhao, Q., et al. Drp1 regulates transcription of ribosomal protein genes in embryonic hearts. J Cell Sci. 2022. 135(4):jcs258956.
- Knight, R., et al. Dietary inflammation score is associated with perceived stress, depression, and cardiometabolic health risk factors among a young adult cohort of women. Clin Nutr ESPEN. 2022. 51:470-477.
- Hazra, S., et al. Mesenchymal stem cell bioenergetics and apoptosis are associated with risk for bronchopulmonary dysplasia in extremely low birth weight infants. Sci Rep. 2022. 12(1):17484.
- Gupta, P., et al. Genetic impairment of succinate metabolism disrupts bioenergetic sensing in adrenal neuroendocrine cancer. Cell Rep. 2022. 40(7):111218.
- Graham, Z.A., et al. SS-31 does not prevent or reduce muscle atrophy 7 days after a 65 kdyne contusion spinal cord injury in young male mice. Physiol Rep. 2022. 10(10):e15266.
- Chocron, E.S., et al. Mitochondrial TrxR2 regulates metabolism and protects from metabolic disease through enhanced TCA and ETC function. Commun Biol. 2022. 5(1):467
- Cedillo, Y.E., et al. Body fat percentage assessment using skinfold thickness agrees with measures obtained by DXA scan in African American and Caucasian American women. Nutr Res. 2022. 105:154-162
- Butts, B., et al. Racial Differences in XO (Xanthine Oxidase) and Mitochondrial DNA Damage-Associated Molecular Patterns in Resistant Hypertension. Hypertension. 2022. 79(4):775-784.
2021 [15-18]
- Fisher, G., et al. Sex and race contribute to variation in mitochondrial function and insulin sensitivity. Physiol Rep. 2021. 9(19):e15049
- Hinshaw, D.C., et al. Hedgehog Signaling Regulates Metabolism and Polarization of Mammary Tumor-Associated Macrophages. Cancer Res. 2021. 81(21):5425-5437.
- Nam, H., et al. The TGF-β/HDAC7 axis suppresses TCA cycle metabolism in renal cancer. JCI Insight. 2021. 6(22):e148438.
- Pitale, P.M., et al. Tribbles Homolog 3 Mediates the Development and Progression of Diabetic Retinopathy. Diabetes. 2021. 70(8):1738-1753.
2020 [19-23]
- Warren, J.L., et al., Exercise Effects on Mitochondrial Function and Lipid Metabolism during Energy Balance. Medicine and science in sports and exercise, 2020. 52(4):827-834
- Turbitt, W.J., et al. Obesity and CD8 T cell metabolism: Implications for anti-tumor immunity and cancer immunotherapy outcomes. Immunol Rev. 2020. 295(1):203-219.
- Sertie, R., et al. In utero nutritional stress as a cause of obesity: Altered relationship between body fat, leptin levels and caloric intake in offspring into adulthood. Life Sci. 2020. 254:117764.
- Saini, V., et al., Hydrogen sulfide stimulates Mycobacterium tuberculosis respiration, growth and pathogenesis. Nat Commun, 2020. 11(1): p. 557.
- Cedillo, Y.E., et al., Physiological Significance of Discrimination on Stress Markers, Obesity, and LDL Oxidation among a European American and African American Cohort of Females. Int J Behav Med, 2020. 27(2):213-224.
2019 [24-32]
- Spurlock, B., et al. New quantitative approach reveals heterogeneity in mitochondrial structure-function relations in tumor-initiating cells. J Cell Sci. 2019. 132(9):jcs230755.
- Ramani, M., et al., Early Life Supraphysiological Levels of Oxygen Exposure Permanently Impairs Hippocampal Mitochondrial Function. Sci Rep, 2019. 9(1): p. 13364.
- Long, Q., et al., Assessing Mitochondrial Bioenergetics in Isolated Mitochondria from Mouse Heart Tissues Using Oroboros 2k-Oxygraph. Methods Mol Biol, 2019. 1966: p. 237-246.
- Hunter, G.R., et al., Relationship between Vo2peak, cycle economy, and mitochondrial respiration in untrained/trained. J Appl Physiol (1985), 2019. 127(6): p. 1562-1568.
- Hanaoka, B.Y., et al., Chronic Inflammation in Rheumatoid Arthritis and Mediators of Skeletal Muscle Pathology and Physical Impairment: A Review. Arthritis Care Res (Hoboken), 2019. 71(2): p. 173-177.
- Farias Quipildor, G.E., et al., Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice. Geroscience, 2019. 41(2): p. 185-208.
- Cui, H., et al., Impairment of Fatty Acid Oxidation in Alveolar Epithelial Cells Mediates Acute Lung Injury. Am J Respir Cell Mol Biol, 2019. 60(2): p. 167-178.
- Cui, H., et al., Long noncoding RNA Malat1 regulates differential activation of macrophages and response to lung injury. JCI Insight, 2019. 4(4).
- Bell, M.B., et al. Adult skeletal muscle deletion of Mitofusin 1 and 2 impedes exercise performance and training capacity. J Appl Physiol. 2019. 126(2):341-353
2018 [33-39]
- Xu, S., et al., E2F1 Suppresses Oxidative Metabolism and Endothelial Differentiation of Bone Marrow Progenitor Cells. Circ Res, 2018. 122(5): p. 701-711.
- Sadahiro, H., et al. Activation of the Receptor Tyrosine Kinase AXL Regulates the Immune Microenvironment in Glioblastoma. Cancer Res. 2018. 78(11):3002-3013.
- Patel, M., et al. Oxalate induces mitochondrial dysfunction and disrupts redox homeostasis in a human monocyte derived cell line. Redox Biol. 2018. 15:207-215.
- Mota MSV,. Deficiency of tumor suppressor Merlin facilitates metabolic adaptation by co-operative engagement of SMAD-Hippo signaling in breast cancer. Carcinogenesis. 2018. 39(9):1165-1175.
- Fruge, A.D., et al., Fingernail and toenail clippings as a non-invasive measure of chronic cortisol levels in adult cancer survivors. Cancer Causes Control, 2018. 29(1): p. 185-191.
- Ederer, K.A., et al., Age- and Genotype-Specific Effects of the Angiotensin-Converting Enzyme Inhibitor Lisinopril on Mitochondrial and Metabolic Parameters in Drosophila melanogaster. Int J Mol Sci, 2018. 19(11).
- Cui, H., et al., IFN Regulatory Factor 2 Inhibits Expression of Glycolytic Genes and Lipopolysaccharide-Induced Proinflammatory Responses in Macrophages. J Immunol, 2018. 200(9): p. 3218-3230.