Submission for Device Trial is required for any clinical trial/research project that involves the use of any type of device (Category A, Category B, Non-Significant Risk, 510K Summary, Wearable Device, PMA, etc.) for which clinical billable activities are provided and billed by the UAB Health System.
“Hospital LOA Requirement: The study information will be submitted by CBR on your behalf to the Hospital Administration for review to determine if a Letter of Agreement (LOA) will be required. Note: for questions regarding Hospital Letter of Agreement, please contact Ron Evans ( raevans@uabmc.edu )
Device Trials: Fill out the appropriate information in the online Redcap application to submit a request for review for studies involving UAB Health System Clinical Billable Services, OnCore Calendar Requests, and/or UAB Health System Clinical Billable Services.
Please note: you will need all required information on hand before submission. Please see the checklist at this link (BlazerID email/password for login) in order to determine needed information for review before you begin your submission.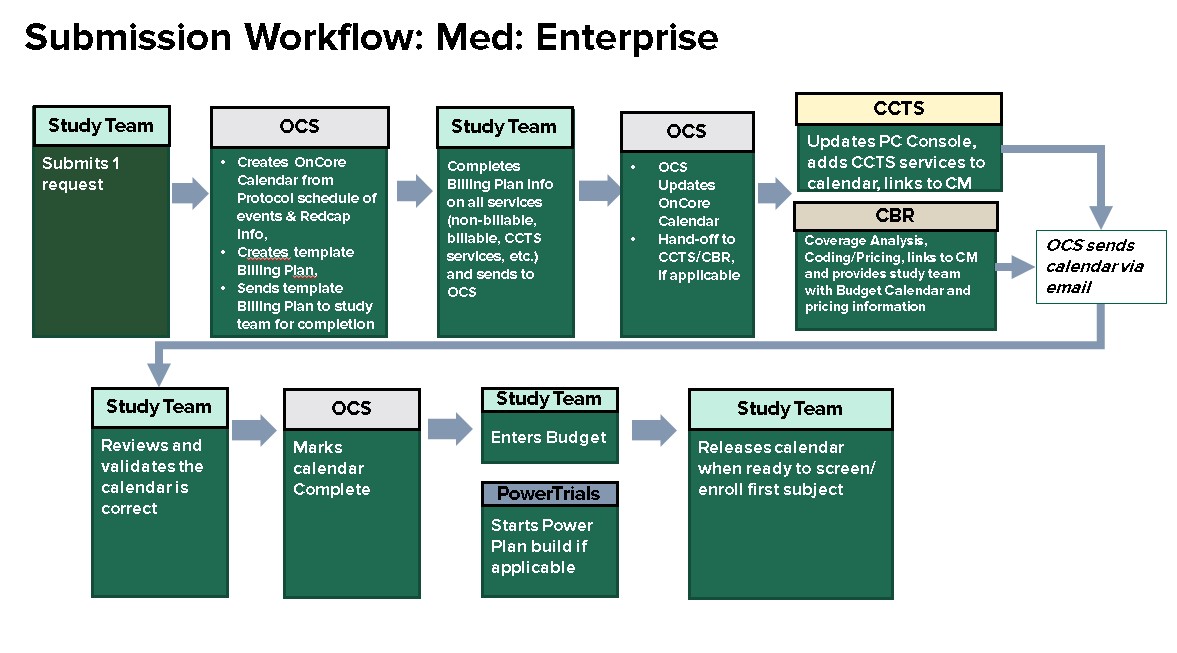
Hospital Review of Clinical Trial Device Workflow
University Hospital Equipment and Supplies Agreement Template, if appropriate
NOTE: If research activities are being provided by an Ancillary Research Area that bills for their services internally - department to department (outside of the UAB Health System), please contact the ancillary research area directly for research pricing.