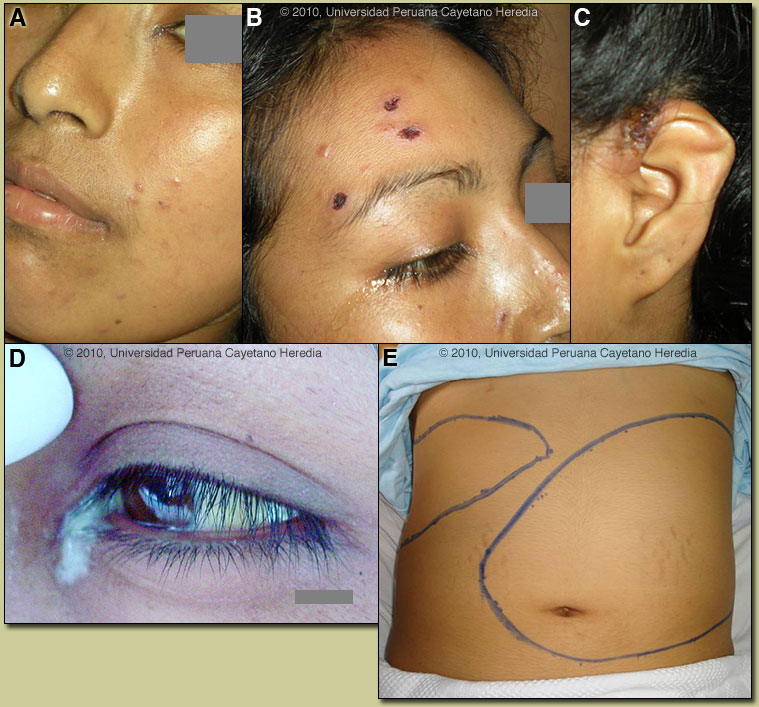
2010 Case #7 |
 |
| Diagnosis: Paracoccidioidomycosis, juvenile form. |
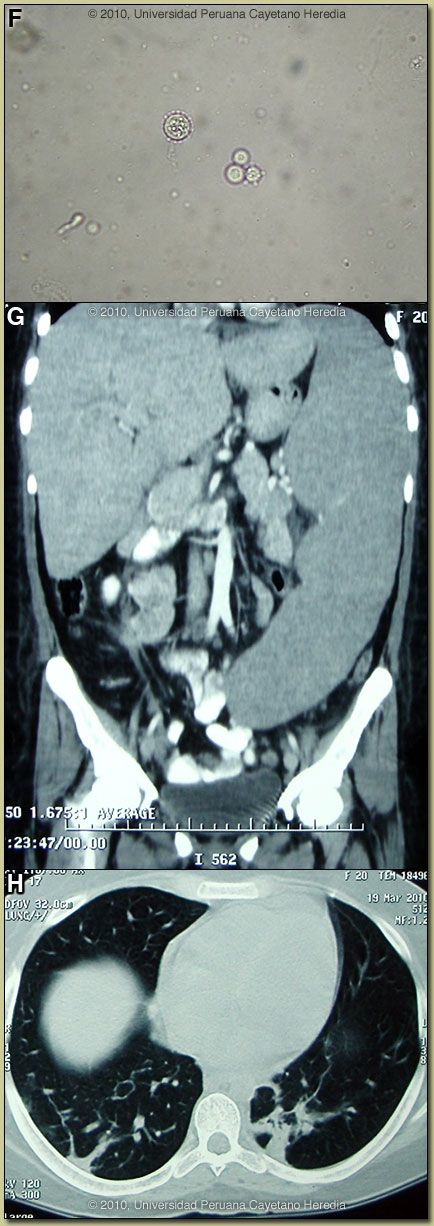 Discussion: Direct KOH preparations of the purulent discharge from the left lacrimal gland, from scrapings of cutaneous lesions and from an aspirate of a cervical lymph node showed spherical cells 10-40 microns in diameter with a thick birefringent cell wall surrounded by several peripheral buds characteristic of Paracoccidioides brasiliensis [Image F]. When completely surrounded by buds a so-called “pilot-wheel” pattern occurs. All cultures were positive for Paracoccidioides brasiliensis. Direct scrapings or aspirates will be positive in the vast majority of cases of paracoccidioidomycosis with cutaneous lesions. Abdominal CT-Scan: non-focal hepatomegaly, massive splenomegaly (24 cm in the longitudinal diameter); and intra-abdominal lymphadenopathy [Image G]. Chest CT-Scan: nodular lesions in both lungs with parenchymal alveolar and interstitial infiltrates [Image H]. Discussion: Direct KOH preparations of the purulent discharge from the left lacrimal gland, from scrapings of cutaneous lesions and from an aspirate of a cervical lymph node showed spherical cells 10-40 microns in diameter with a thick birefringent cell wall surrounded by several peripheral buds characteristic of Paracoccidioides brasiliensis [Image F]. When completely surrounded by buds a so-called “pilot-wheel” pattern occurs. All cultures were positive for Paracoccidioides brasiliensis. Direct scrapings or aspirates will be positive in the vast majority of cases of paracoccidioidomycosis with cutaneous lesions. Abdominal CT-Scan: non-focal hepatomegaly, massive splenomegaly (24 cm in the longitudinal diameter); and intra-abdominal lymphadenopathy [Image G]. Chest CT-Scan: nodular lesions in both lungs with parenchymal alveolar and interstitial infiltrates [Image H].
Records were obtained that juvenile paracoccidioidomycosis had been diagnosed at the time of the initial illness at the age of 12. Treatment with amphotericin B deoxycholate was given for one month followed by irregular itraconazole (100 mg/daily) over several subsequent years. In Perú and Latin America other causes for massive splenomegaly would include visceral leishmaniasis (not present in Perú), schistosomiasis (not present in Perú), chronic brucellosis, hematologic malignancies, and hemophagocytic syndromes. Other causes of severe and potentially massive splenomegaly include EBV infection, infective endocarditis, and mycobacterial infections. Giant splenomegaly with multiple abscesses has been reported in HIV infected patients caused by either tuberculous or non-tuberculous mycobacterial infections. Hyperreactive malarial splenomegaly, previously known as tropical splenomegaly syndrome (TSS), is a complication of chronic malaria. It is not seen in Latin America, as this requires a high level of constant exposure to infective mosquitoes at a degree that only exists in Africa and some South Pacific countries such as Indonesia and Papua New Guinea. The constant antigenic exposure stimulates immune complex deposition throughout the reticuloendothelial system. Disseminated histoplasmosis does not usually cause as massive a splenomegaly. Paracoccidioidomycosis is most often an indolent and relapsing disease causing chronic pulmonary and mucosal manifestations. The chronic form (adult type) of the disease is believed to represent reactivation of latent infection initially acquired via inhalation. The chronic forms represent approximately 94% of all cases in the experience at our institute (94 patients, up to 2001), and approximately 85% in the Brazilian series [Rev Soc Bras Med Trop. 2003 Jul-Aug;36(4):455-9]. See examples of this in Gorgas Cases 2005-12, 2009-06, and 2004-05]. We have also previously shown a case of the chronic progressive form of the disease [Gorgas Case 2003-07]. This current case is representative of the relatively uncommon “juvenile” acute progressive and disseminated form of the disease, which usually (but not always) affects adolescents and young adults. After an initial inhalation event [Clin Infect Dis. 2005 Jan 1;40(1):e1-4] which is usually self-resolving, the reticuloendothelial system is widely affected. Diffuse lymphadenopathy, hepatosplenomegaly, and papular skin lesions with a large burden of yeast forms in all tissues as in our patient are usual clinical manfestations. The course is rapidly progressive over weeks in contrast to months or years for the chronic progressive forms. Early on in juvenile disease, CT imaging of the chest reveals changes consisting of mediastinal and hilar adenopathy and interstitial infliltrates [Semin Respir Crit Care Med. 2008 Apr;29(2):182-97]. The respiratory system and mucosal surfaces are not usually involved in the clinical manifestations of the acute juvenile disease as they are in the chronic forms. However, in the present case the chest CT is abnormal and ongoing pulmonary involvement has been uncommonly reported and is not surprising given this patient’s longstanding disease. The juvenile patients manifest with an antigen specific depressed cell mediated immune response to paracoccidioidal antigens in vitro and with negative skin tests. Responsiveness to mitogens and other antigens is normal. In vitro, patients have a predominant Th2 cytokine pattern. Sulfonamides, ketoconazole, itraconazole, and amphotericin B are all effective therapies. Amphotericin should be reserved for severe cases such as this one. For milder cases, itraconazole 100-200 mg/day for 6-9 months is regarded as the treatment of choice for both the juvenile and chronic forms when it is available and affordable. Relapses are common with less than 6 months therapy and expert opinion is now that 1 year is not necessary. In the developing-world setting, ketoconazole is likely equally effective and is usually less than half the cost. However, 12 months of therapy with ketoconazole is generally recommended. In severe cases with a high yeast burden such as in our case, the practice is to induce such patients with amphotericin B for at least 10 days, then switch to oral itraconazole 200mg per day, to complete a total duration of at least 9 months. In this severe and relapsing case, the plan is to give amphotericin B for one month, then one-year with itraconazole 200 mg/d, followed by lifelong suppressive treatment (100 mg/d). |