 |
Gorgas Case 2016-05 |
 |
|
The patient was seen on the wards of the Cusco Regional Hospital.
 History: 26-year-old male with severe motor vehicle trauma 2 months before current admission including a brachial plexus injury. 5 weeks prior to this admission he was discharged with paralysis and severe neuropathic pain of the left arm for which he was prescribed dexamethasone, which he took for an uncertain period. Approximately 1 month before admission he developed progressively worsening nausea and vomiting associated with anorexia, abdominal distension, and weight loss of about 12 kg. 3 weeks before admission lower extremity edema and severe fatigue developed. Three days before admission, nausea, vomiting, and sudden onset of severe hematemesis led to urgent re-admission.
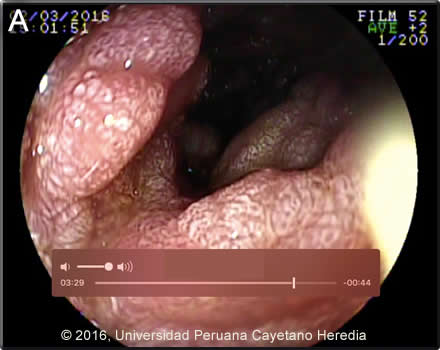
Epidemiology: The patient was born and lives in Kiteni in the lowland jungle region of Cusco Department. Works operating heavy machinery in both the jungle and highland regions of Cusco. He denies alcohol or drug abuse. He has one stable female partner for the last 4-5 years and denies paying for sex. Physical Examination: Blood pressure: 80/46. HR: 129 RR: 24 O2 saturation: 86% (FiO2 0.21) Severe pallor and in obvious distress, non-icteric. HEENT: normal oral, nasal and pharngel mucosa. Skin: several well healed scars on the head and extremities, no draining wounds or sinuses. No spider angiomas. No petechiae or ecchymosis. Abdomen: Mild distension, soft non-tender. No hepatosplenomegaly. Musculoskeletal: 1+ edema around the ankles. Neurologic: Flaccid paralysis of the left arm with no deep tendon reflexes. Upper endoscopy showed 2 Mallory-Weiss tears of the esophageal mucosa with no active bleeding, a normal gastric mucosa, and the duodenal findings shown in Image A. Laboratory Examination (on admission): Hemoglobin: 3.6 g/dL (MCV: 82 fl). Hematocrit: 10.2%. WBC: 25.8x103/mm3 (81 neutrophils, 2 eos). AST: 17 U/L, ALT: 18 U/L, Alkaline phosphatase: 152 U/L. Total protein: 3.1 g/dL. Albumin: 1.6 g/dL, HIV rapid test: Negative. HBsAg: Negative. CXR: normal.
|
|
Diagnosis: Strongyloides stercoralis hyperinfection syndrome. HTLV-1 infection.
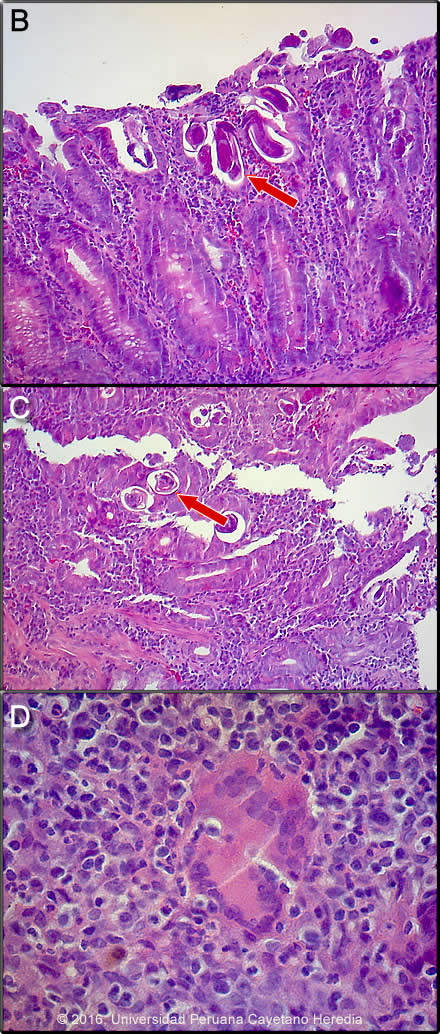
 Discussion: Duodenal endoscopy (Image A) showed severe swelling of the mucosa throughout the third portion of the duodenum with areas of stenosis [see Gorgas Case 2007-04 for an image of endoscopically visible larvae with strongyloides duodenitis]. A duodenal biopsy (Images B,C,D) showed duodenal mucosa with erosion, ulceration, necrosis and severe acute and chronic inflammation with tuberculoid graanuloma formation with multinucleated giant cells, vascular congestion, and hemorrhage. The intestinal lumen and crypts show multiple parasitic structures compatible with rhabditiform larvae of Strongyloides stercoralis. Other nematodes where larvae or adults may be seen invading the intestinal wall include Capillaria philippinensis and Trichinella spiralis. HTLV-1 ELISA and Western blot were positive. Transmission is by breastfeeding; we have yet to test the mother or siblings. Stool examination was not performed but large numbers of larvae would be expected (Clin Infect Dis. 2009 Oct 1;49(7):1094-5, 1132-3. doi: 10.1086/605628) based on our experience. Sputum is variably positive in these cases. In addition to disseminated strongyloidiasis, a number of conditions are associated with HTLV-1 infection [see Lancet Infect Dis. 2007 Apr;7(4):266-81 for a detailed discussion], including crusted (Norwegian) scabies [see Gorgas Case 2008-08], tropical spastic paraparesis (TSP, also called HAM – HTLV-1 associated myelopathy) [see Gorgas Case 2002-08], infective dermatitis [see Gorgas Case 2004-07], adult T-cell leukemia/lymphoma (ATLL) [see Gorgas Case 2009-11], autoimmune disease including uveitis, Sjögrens, arthropathy, polymyositis, and thyroiditis. Associations with bronchiectasis [see Clin Infect Dis. 2012 Jan;54(1):43-50] and paracoccidiodomycosis [see Clin Infect Dis. 2010 Jul 15;51(2):250-1] are recently described. This patient did not have any neurological findings other than those related to the injury. More than 90% of individuals with HTLV-1 infection remain asymptomatic for life. Worldwide, approximately 0.3-4.0% develop TSP and 1-5% develop ATLL. Although the pathogenetic mechanisms of these two major complications likely differ, the low rates of each means that it is rare to see both sequelae in the same patient. In Perú 86% of patients who present with strongyloides hyperinfection are HTLV-1 positive [see Am J Trop Med Hyg. 1999 Jan;60(1):146-9]. The prevalence of HTLV-1 in South America is generally underappreciated, normally being associated with Japanese and Caribbean populations. In Perú, the disease is highly endemic (2-3% seropositivity) in Andean areas of the country in Quechua populations who have had no contact with Japanese immigrants to the country. Data indicates that the area around Kiteni where this patient is from has a similar prevalence due to migration over the years from the Andes. Other South American countries with significant rates of HTLV-1 include Brazil, Colombia, and Ecuador. We have seen over 800 infected families to date at the Tropical Medicine Institute in Lima. Steroids alone may induce strongyloides hyperinfection but usually after several weeks of therapy. This patient illness developed within days of commencing dexamethasone, something we have commonly seen in those with HTLV-1 infection. Strongyloides stercoralis has a complex life cycle. Three separate developmental stages play a role in the life cycle: adult worms, rhabditiform larvae, and filariform (infectious) larvae. Three alternative clinically important modes of replication exist. In the internal sexual cycle the adult female, resident in the small intestine, lays small numbers of eggs that hatch almost immediately. The resulting rhabditiform larvae are passed in the stool, mature in the soil to infective filariform larvae, and penetrate the skin of a new human host. After migration through the lung, the larvae crawl over the glottis, are swallowed and complete development in the jejeunum. In the free-living cycle, rhabditiform larvae may instead develop in the soil into sexually active adults, thus creating an environmental reservoir of adult worms. Each generation of this external sexual cycle includes a new cohort of infectious filariform larvae ready to re-enter the parasitic cycle in humans. Finally, in the autoinfective cycle, infectious filariform larvae develop from rhabditiform larvae while still in the intestine. Penetration of the colon or the anal skin by filariform larvae allows re-infection of the same host. The autoinfective cycles, which normally result in low-grade chronic infection in normal hosts, should be distinguished from the hyperinfection that occurs in immunocompromised hosts as a result of massive potentially fatal autoinfection with dissemination of larvae to numerous distant organs. The following countries have areas of high (>20%) prevalence of strongyloides: Argentina, Ecuador, Venezuela, Peru and Brazil (Epidemiol Infect. 2015 Feb;143(3):452-60. doi: 10.1017/S0950268814001563). Clinically, strongyloides infection is often asymptomatic. Purely intestinal infection may cause mild to severe abdominal symptoms. Adult females in the small intestinal mucosa may cause mild to severe inflammation. In autoinfective cycles, migrating larvae may cause serpiginous skin lesions (larva currens), especially around buttocks, or lung inflammation. Opportunistic behavior in immunocompromised hosts with hyperinfection may result in wide dissemination to extraintestinal organs, including the central nervous system, pulmonary hemorrhages, sepsis, and death. Inflammation and necrosis may occur in any organ and massive GI bleeding, toxic megacolon, and intestinal perforation may occur. Predisposing factors include malnutrition, corticosteroid therapy, malignancy, and HTLV-1 (but not HIV) infection. Patients with strongyloides hyperinfection may have eosinophilia in the early stages of disseminated disease, but are most often eosinopenic by the time of clinical presentation. In our institute over 50% of patients who present with strongyloides hyperinfection are individuals born in the highlands where HTLV-1 is prevalent who then acquire strongyloides in the jungle lowlands. Due to likely poor absoption in this patient with intestinal edema and low albumin, oral ivermectin (200ug/kg/d) was administered for every other day for 5 doses. He should be retreated every 15 days at least 3 times as per our practice [see Trans R Soc Trop Med Hyg. 2008 Apr;102(4):314-8]. Ivermectin shows clear superiority over albendazole for treatment of S. stercoralis (Cochrane Database Syst Rev. 2016 Jan 18;1:CD007745. doi: 10.1002/14651858.CD007745.pub3) We would like to thank, Drs. Miguel Cabada and Clinton White, Gorgas Course Visiting Professors from the University of Texas Medical Branch in Galveston for providing this case and for helpful discussions. |