Click here to view the full press kit that includes photos, video interviews and infographics.
Learn more about UAB's Xenotransplant program here.
www.uab.edu/news-csvi/xenotransplant
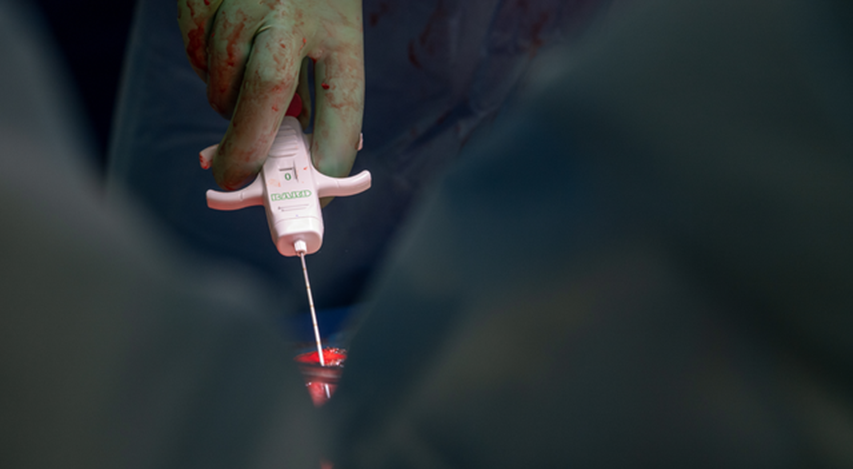
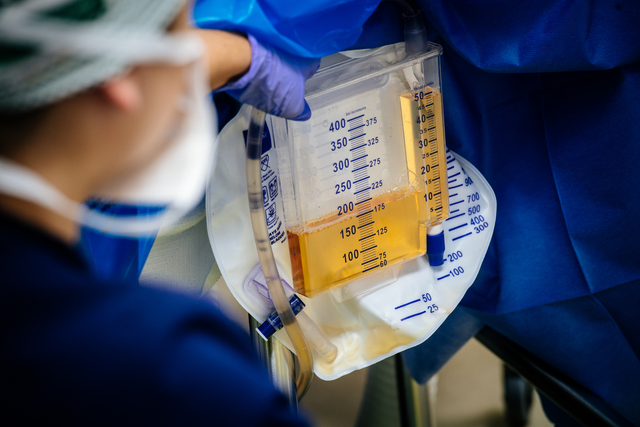 UAB Registered Nurse Loren Brook Eichelberger measures the urine output (175 cc) from the transplanted genetically modified pig kidney as part of the 2023 JAMA Surgery study.
UAB Registered Nurse Loren Brook Eichelberger measures the urine output (175 cc) from the transplanted genetically modified pig kidney as part of the 2023 JAMA Surgery study.
Photography: Steve WoodFor the first time in a human, genetically modified pig kidneys provided “life-sustaining kidney function” during the course of a planned seven-day study — all while using current standard-of-care immunosuppression drugs.
The peer-reviewed findings from a study conducted in Feb. 2023, published today in JAMA Surgery, extend another pioneering University of Alabama at Birmingham pre-clinical human research model study in a recipient experiencing brain death. It also advances the science and promise of xenotransplantation as a therapy to potentially cure end-stage kidney disease — just as a human-to-human allotransplant can — and addresses the critical worldwide kidney organ shortage crisis.
“It has been truly extraordinary to see the first-ever preclinical demonstration that appropriately modified pig kidneys can provide normal, life-sustaining kidney function in a human safely and be achieved using a standard immunosuppression regimen,” said UAB transplant surgeon scientist Jayme Locke, M.D., director of UAB’s Comprehensive Transplant Institute in the Marnix E. Heersink School of Medicine and lead author of the JAMA Surgery paper. “The kidneys functioned remarkably over the course of this seven-day study. We were able to gather additional safety and scientific information critical to our efforts to seek FDA clearance of a Phase I clinical trial in living humans and hopefully add a new, desperately needed solution to address an organ shortage crisis responsible for tens of thousands of preventable deaths each year.”
 Jayme Locke, M.D., director of the UAB Comprehensive Transplant Institute and lead author of the JAMA study
Jayme Locke, M.D., director of the UAB Comprehensive Transplant Institute and lead author of the JAMA study
Photography: Steve Wood
The published findings are another major xenotransplant achievement by Locke and her Heersink School of Medicine team. They come 19 months after last year’s groundbreaking peer reviewed, published UAB xenotransplant research study in which genetically modified pig kidneys were successfully transplanted into a recipient after brain death.
Now, the research has taken another significant step forward.
The study was conducted using the Parsons Model, a pre-clinical human brain death model developed at UAB to evaluate the safety and feasibility of pig-to-human kidney xenografts, or transplants, without risk to a living human. It is named for transplant pioneer Jim Parsons, an organ donor whose family generously donated his body to advance xenotransplant kidney research. Parsons’ gift led to the first clinical grade pig kidney xenograft transplant into a human and helped pave the way for future pig kidney-to-living human transplantation.
The current research also was conducted in a person who indicated to his family that he wanted his body donated for research upon his death.
Study details
The 52-year-old study subject for this research, not named at the request of his family, lived with hypertension and stage 2 chronic kidney disease, which affects more than one in seven U.S. adults, or an estimated 37 million Americans. As part of this study, the subject had both of his native kidneys removed and dialysis stopped, followed by a crossmatch-compatible xenotransplants with 10 gene-edited pig kidneys, or UKidney™.
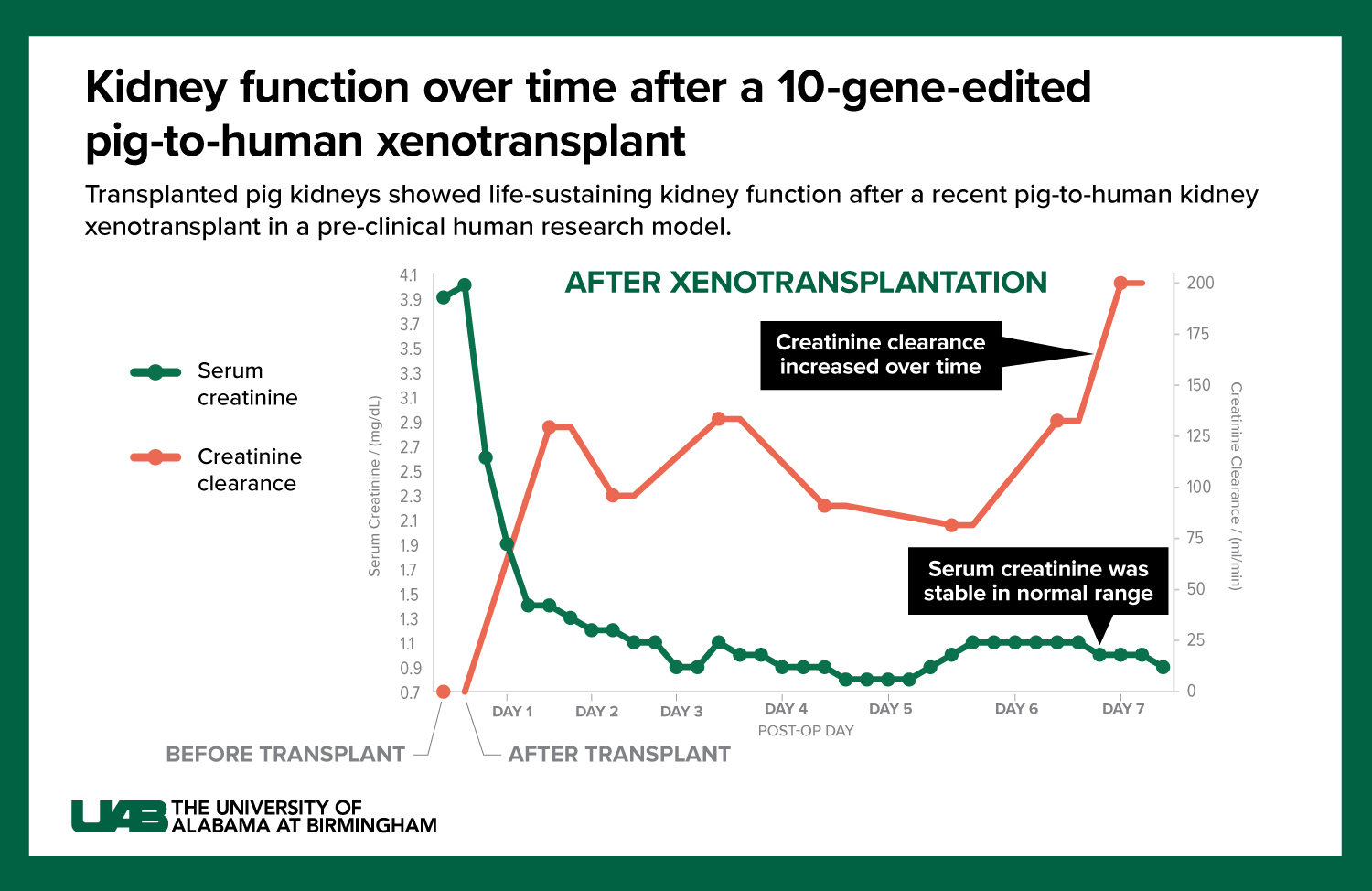
Graphic by: Jody Potter
-
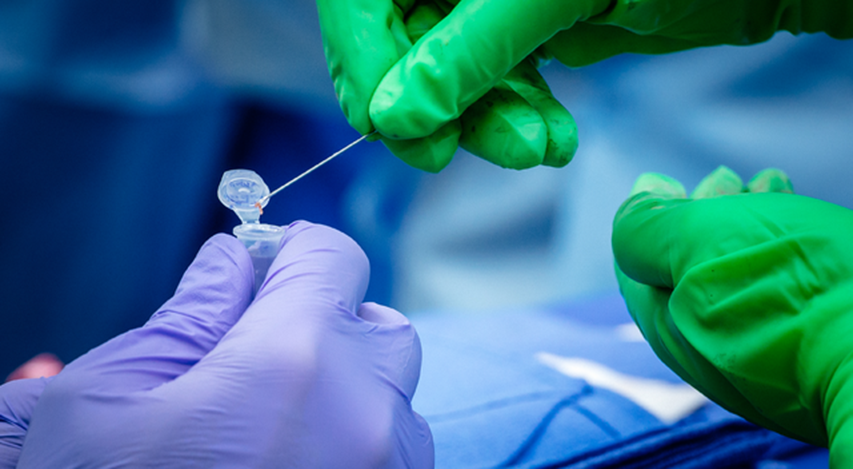
This close-up photo shows UAB Dr. Paige Porrett (green gloves) placing a piece of a biopsied, genetically engineered pig kidney into a vial held by UAB Transplant Researcher Gavin Baker.
Photography: Steve Wood
-
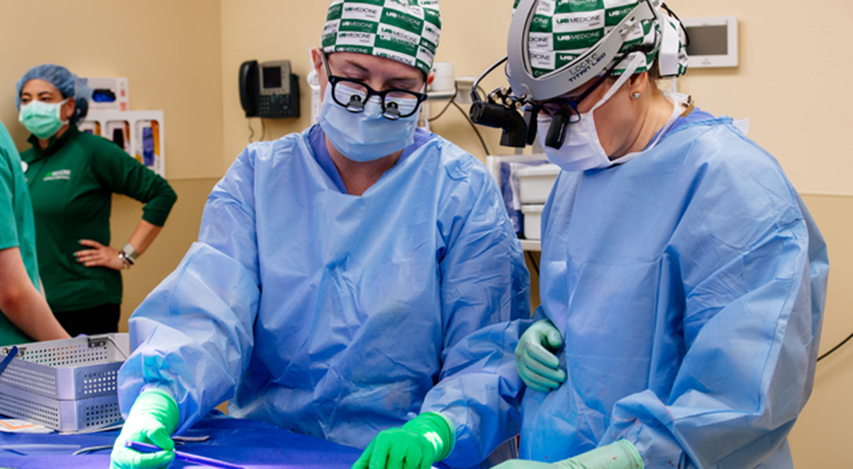
UAB Dr. Paige Porrett, M.D., Ph.D., Associate Professor of Surgery and Dr. Jayme Locke, MD, Director, UAB Division of Transplantation, work through a volumetric equation to measure the kidney growth as part of the 2023 JAMA Surgery study.
Photography: Steve Wood
-
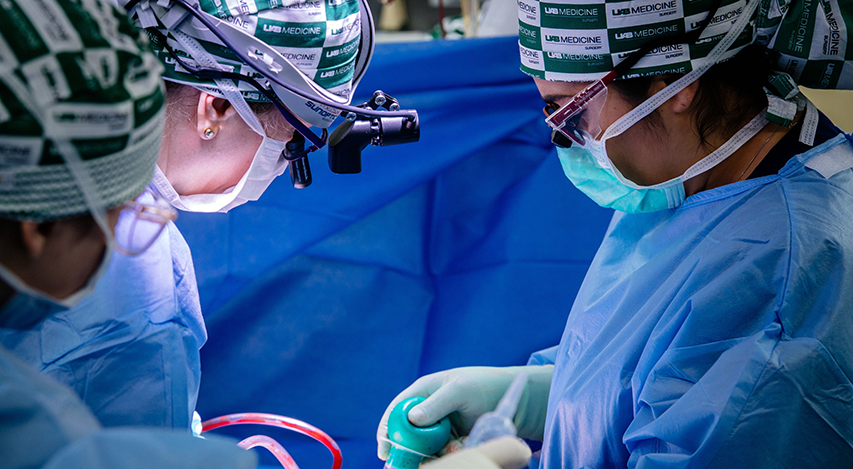
From left to right: Sandi Minor, RN, Registered Nurse, Perioperative Services; Dr. Jayme Locke, MD, Director, UAB Division of Transplantation; and Natalie Budd, RN, Registered Nurse, Perioperative Services, transplant a genetically modified pig kidney in a pre-clinical human brain death model developed at UAB. The transplant was part of UAB’s 2023 JAMA Surgery study.
Photography: Steve Wood
-
Dr. Jayme Locke, M.D., Director, UAB Division of Transplantation, takes a biopsy of the transplanted genetically modified pig kidneys as part of the 2023 JAMA Surgery study.
Photography: Steve Wood
-
UAB's Xenotransplant team. (From left) Andrew "Drew" Shunk, Director of Clinical Services, Legacy of Hope; Dr. Jayme Locke, MD, Director, UAB Division of Transplantation; and Dr. Vineeta Kumar, MD, Professor, Nephrology, review and discuss xenotransplant patient biopsy lab results for the 2023 JAMA Surgery study.
Photography: Steve Wood
These studies are supported by biotechnology pioneer United Therapeutics Corporation, which awarded a grant to UAB to launch the innovative xenotransplantation program. Revivicor, Inc., a subsidiary of United Therapeutics, provided the genetically modified pig that was the source of the investigational xenotransplant kidneys called UKidney™. The pigs are raised in a secure, pathogen-free facility by UAB animal care experts and are regularly tested for porcine cytomegalovirus (CMV) and porcine endogenous retroviruses (PERV), including PERV-C. The pig used in this study tested negative for PERV-C and CMV.
“We are grateful for our partners at Revivicor and United Therapeutics and their tireless efforts on this project,” said UAB Heersink School of Medicine Dean and Senior Vice President of Medicine Anupam Agarwal, M.D. “There is still important work to do, but we have made tremendous progress together.”
UAB President Ray L. Watts, M.D., says partnerships and sacrifices inside and outside of UAB have been critical.
“Xenotransplantation is a pioneering treatment that has the potential to provide a sustainable supply of life-saving organs and save tens of thousands of lives of people around the world, and it takes a village,” Watts said. “We are proud of Dr. Jayme Locke and our expert teams who have been so thorough in designing and carrying out these studies in a way that advances science. We also remain in awe and eternally grateful for the individuals and families who have given of themselves and their loved ones to participate in these studies that will improve and save lives.”
Parsons Model studies advance field
 Click image to enlarge.
Click image to enlarge.
Graphic by: Jody PotterThis is the third study in UAB’s Parsons Model program, and the second one to be peer-reviewed and published (Locke’s team is in the process of publishing results from another Parsons Model study). Locke and her research team demonstrated in an initial study last year the model’s significant potential to propel the xenotransplantation field forward and help solve the kidney shortage crisis.
Each of the model’s studies have built upon the other and answered critical questions on the viability of pig kidney xenotransplantation. Specifically, for the two peer-reviewed, published studies:
- The original American Journal of Transplantation study from 2022, named after Parsons, marked the first time a pig-to-human cross match was developed and validated. That step was important because federal requirements for human-to-human allotransplantation include a crossmatch and tissue compatibility prior to proceeding with a transplant.
- In today’s study published in JAMA Surgery, the decedent was stable when he presented for the study which allowed Locke’s team to follow the investigation for seven days. Because the study subject was stable, and the kidneys were in a favorable environment, there was no delay of kidney function — something that wasn’t the case in the first two studies. “In this third study, we were able to demonstrate urine output within four minutes of the kidneys being re-perfused,” Locke said. “In fact, in the first 24 hours these kidneys made over 37 liters of urine. It was really a remarkable thing to see.”
As in the two previous studies, each stage of this third decedent xenotransplant study approximated the steps that would be taken in a Phase I clinical trial of xenotransplantation in living humans.
“In each instance, we have been able to test our operations,” said Locke, director of transplantation at the UAB Comprehensive Transplant Institute. “The pig kidneys came from a pig maintained in a pathogen-free facility. They were flushed and packaged using the same standard operating procedures we use in human-to-human transplantation. They were transported to our transplant center and transplanted in the exact same way you would in a living person.
“At each of those steps, we were able to test that we do have the correct standard operating procedures in place and that we’re able to operationalize this in a meaningful, safe way. That’s the ultimate goal. We want to achieve xenotransplant in a safe and efficacious way. We’re very hopeful that these data will provide further evidence that xenotransplantation is a viable and achievable solution to the organ shortage crisis that causes thousands of preventable deaths each year. The gap between supply and demand is that vast.”
Gene editing in pigs to reduce immune rejection has made organ transplants from pigs to humans possible, an advancement that could offer help to thousands of people who face organ failure, disease or injury. The pig kidneys used in the UAB studies come from pigs with 10 specific genetic modifications, each of which is intended to make the organs suitable for transplant into the human body.
The natural lifespan of a pig is 30 years, they are easily bred, and they have organs of similar size to humans. Genetically modified pig kidneys have been extensively tested in non-human primates. The addition of UAB’s preclinical human research model— the Parsons Model — now provides important information about the potential safety and efficacy of kidneys in human transplant recipients, including in clinical trials.
“Non-human primate research cannot answer whether the humanized pig kidneys will serve as a destination or bridge therapy,” Locke said. “Only human xenotransplantation can fully test this.”
The critical need
Kidney disease kills more people each year than breast or prostate cancer, according to the National Institute of Diabetes and Digestive and Kidney Diseases. Although transplantation is the gold standard treatment for end-stage kidney disease, fewer than 25,000 kidney transplants are performed each year in the United States and more than 90,000 people are on the transplant waiting list. More than 800,000 Americans are living with kidney failure and 240 Americans on dialysis die every day. Most never make it to the waiting list, and far too few human organs are available to put a dent in that number.
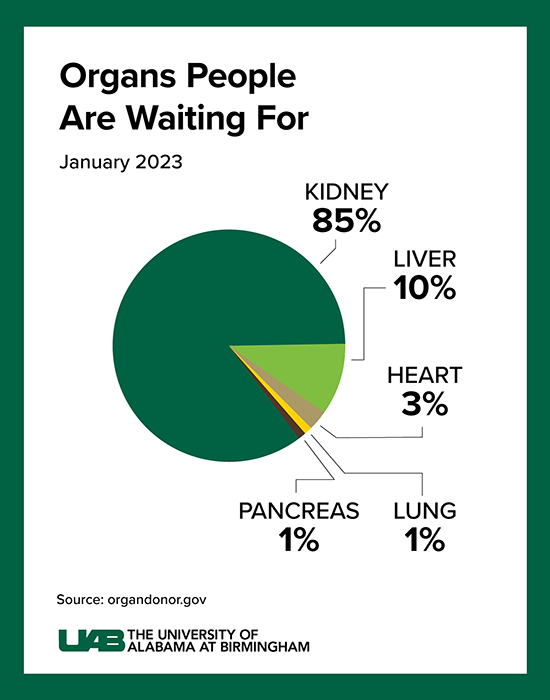 Click image to enlarge.
Click image to enlarge.
Graphic by: Jody PotterThe wait for a deceased donor kidney can be as long as five years, and in many states, it is closer to 10 years. Almost 5,000 people per year die waiting on a kidney transplant.
Many of these deaths could be prevented if an unlimited supply of kidneys were available for transplant.
Decades of human-to-human kidney transplants have shown that a kidney recipient has a greater longevity and quality of life than a kidney-disease person on chronic dialysis. About 5 to 15 percent of dialysis patients die yearly, and the eight-year survival rate is only about 35 percent.
All of this shows a radical solution is needed for the organ supply crisis, Locke says. If many people are compatible for a xenograft from a pig, those xenotransplants — paired with human-to-human allotransplantation — could potentially eliminate the wait for a kidney.
“The concept of being able to have an organ imminently available, waiting for the person who needs it, is the type of radical solution that we’ve needed for a long time in transplantation — it’s just remarkable to think about,” Locke said. “These families have made this possible. In each of these studies we have completed at UAB, these families have made the brave decision to allow their loved one to be considered as a donor for the purposes of both transplantation and research. To have the courage to say we want our loved one to have this legacy and move things forward for countless others is just extraordinary. Without question, their loved one’s legacies are sealed. And it is very clear to me that this work is going to be pivotal. It is going to be what gets us across that finish line to move this into living humans.”
“UAB has long been a global leader in kidney transplantation, and we have invested resources very strategically in addressing the organ shortage crisis,” Watts said. “With yet another historic breakthrough, the Heersink School of Medicine and our partners have dramatically advanced the field of xenotransplantation and have demonstrated there is the very real possibility of a future with a sustainable supply of life-saving organs and brings hope to patients worldwide.”
This xenotransplantation study was led by the Division of Transplantation in the Department of Surgery, in close partnership with Legacy of Hope. Other key contributors to this work have included members of the Division of Infectious Diseases and the Division of Nephrology in the Department of Medicine; the Department of Anesthesiology and Perioperative Medicine; and the Department of Pathology. In addition to these specific contributors, the xenotransplantation program at UAB encompasses multiple departments and divisions of the Heersink School of Medicine and is also supported by numerous administrative and regulatory departments.