Displaying items by tag: cancer
If so, they could be a part of a study that looks at the long-term effects of receiving the COVID-19 vaccine. Healthy children or children with conditions affecting their immune systems are eligible to enroll. Blood samples and nasal swabs will be collected every 2 months for one year. An echocardiogram and EKG will also be required at the second visit. Compensation for participation is available. To learn more, contact the UAB study team: 205-638-2629 or mlpurser@uabmc.edu
Published in
Clinical Trials
Do you identify as an individual living with cancer and part of the LGBTQIA+ community or a care partner of someone in this community? A research lab at the University of Alabama at Birmingham is looking to learn about the day-to-day experiences of LGBTQIA+ cancer survivors and their care partners. We are interested in how individuals manage difficult situations related to their respective roles in their relationships. Individuals living with cancer often encounter stressful situations that can impact their mood and any additional stressors from living as a sexual or gender minority with a lack of resources adds an increased risk of experiencing stressful situations. The goal of this work is to develop resources that better support the well-being of LGBTQIA+ individuals living with cancer and their care partners. This study will take place FULLY online with completion of daily surveys. All participants will be compensated. To learn more about our study contact us at starlab@uab.edu
Published in
Clinical Trials
Clinical trial evaluating a novel, non-invasive radiation treatment for ventricular tachycardia refractory to medication and catheter ablation The UAB Departments of Radiation Oncology and Cardiology are looking for patients with ventricular tachycardia (VT) not controlled by medications and catheter ablation. The trial will allow patients suffering from VT despite standard of care regimens to undergo non-invasive cardiac radioablation (CRA; a type of stereotactic body radiotherapy) to the area of the heart responsible for their symptoms. Stereotactic body radiotherapy is delivered with a device called the linear accelerator, which is primarily used to treat patients with cancer. UAB is one of the first centers in the U.S. to obtain a Food and Drug Administration (FDA) investigational device exemption for use of the linear accelerator in this capacity on a phase I/II trial. The study is being conducted in collaboration with the Washington University School of Medicine in St. Louis, a center which pioneered this promising technique in the U.S. For more information on the RAD 1901 trial, please contact Adelyn Gillon, clinical research coordinator, at 205.975.3019 or agillon@uabmc.edu" contenteditable="false">agillon@uabmc.edu.
Published in
Clinical Trials
BAPS is a new Breast Cancer Recovery study opening at the O’Neal Comprehensive Cancer Center. As part of our focus on healthy recovery from cancer and its treatment, we try to offer programs that will support women in their recovery. We are studying a new program that is designed to help women find ways to be active and productive in all areas of life including managing work and employment, taking care of their home and family, and doing healthy activities such as exercise, leisure, hobbies, and socializing. The 9-session program happens over the telephone, at times that are convenient for you. If you would like to learn more about the study, please call 205-996-0384
Published in
Clinical Trials
The ROME study is designed to discover how exercise affects the gut microbiome and fatigue in breast cancer survivors. This study includes 10 weeks of supervised, individual exercise sessions with exercise specialists. The study is funded by the National Cancer Institute. Participants must be between the ages of 18 and 70 with a history of stage I, II or III breast cancer within the last five years. For more information, contact the Cancer Lifestyle Team at (205) 934-8821 or email moveforward@uab.edu. All information is confidential and participation is free.
Published in
Clinical Trials
The wisdom Study wants to apply modern medical science to breast cancer screening by determining if personalized screening is better than annual mammography and learning how to improve detection while reducing over-diagnosis and false-positive readings. Women ages 40-74, with no history of breast cancer or ductal carcinoma in situ (DCIS), are asked to join. Participation can mostly be done from home, and there are no additional medical visits outside routine visits required. Please register at www.wisdomstudy.org
Published in
Clinical Trials
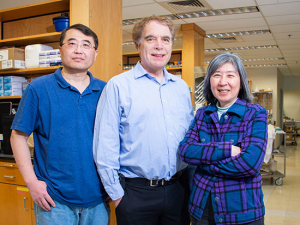
Noha Sharafeldin, MBBCh, Ph.D., of the Institute for Cancer Outcomes and Survivorship, used UAB’s supercomputer to identify biomarkers linked with cognitive impairment in patients who received a blood or marrow transplant. She’s also testing a way to repair the damage.
Published in
Discoveries & Innovations
We are conducting a study to examine the naturally occurring bacteria in the gut of women and how behaviors like diet and stress may affect the presence of bacteria in the gut. Participation includes completing questionnaires, basic body measurements, and providing samples of blood, stool, and saliva. Compensation is provided. For more information contact Sh’Nese Townsend at 205-934-1715 or stownsend@uabmc.edu
Published in
Clinical Trials
Are you a breast cancer survivor having difficulty walking? Do you have any lower-body pain that is restricting your mobility? If so, you may be interested in taking part in an exercise training intervention. UAB researchers are looking for overweight women - age 18-70 with a body mass index between 30-45 kg/m2 - with a history of breast cancer to participate in a novel exercise training study. Volunteers will be asked to attend two assessment sessions: an initial assessment and after 8 weeks of exercise to collect information about your health status. Further eligibility screening will be conducted by telephone. Volunteers must reside in or near Jefferson County. The study will evaluate a novel exercise training strategy designed to mimic exercise at higher altitude to support improved health and mobility among breast cancer survivors. Contact Dr. Stephen J. Carter, 205-975-0269 or carters@uab.edu; or the UAB Exercise and Cancer Research Team, 205.934.5466 or moveforward@uab.edu; to learn more.
Published in
Clinical Trials
If you are experiencing extreme tiredness and fatigue even after your cancer treatment ends, you may be eligible to participate in a new mind/body research study. To qualify for this study, you must be a Stage II, Stage III or Stage IV cancer survivor who has completed all cancer treatment at least 6 months ago and within the last 10 years. Compensation for time and travel will be provided. For more information, please call 205-934-6326.
Published in
Clinical Trials
We are inviting you to participate in the D.I.A.L. physical activity program for 12 weeks at no cost to you. What is it? Receive 12-weeks of physical activity information over the phone. Who can join? Any healthy adult age 21 or older and you do not have to currently be physically active to participate. Why be involved? Contribute to cancer prevention research and receive compensation for your involvement. Call 205-934-0504 to see if you qualify for the program.
Published in
Clinical Trials
Unique study investigates what tools survivors need to engage in regular exercise after treatment
Published in
Get Involved