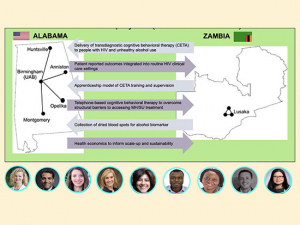
Displaying items by tag: eye health
This is a great opportunity for all to gain experience participating in a clinical research study. All participation would not only contribute to advancing our understanding of perceptual learning and how the brain processes vision, but also holds the potential to improve outcomes for those with central vision loss.
If you or someone you know may be interested, please consider participating.
For more information or to express interest, please email mcmaxwell@uabmc.edu or text or call 205-410-4041, or visit go.uab.edu/brainstudy to sign up!
Thank you for your time and consideration!
If you are interested in participating in this research and want to know more about the study, kindly follow this link to fill out our recruitment survey.
You may be eligible to take part in the SYL1001_V Study if:
• You are 18 years or older
• You have a diagnosis of Sjögren’s
• Other entry criteria apply and your eligibility will be determined by
the study staff
If you decide to take part in the study, your participation could last approximately 3.5 months and consist of 5 to 6 visits to the study center. You will be reimbursed for each completed visit.
Special contact lenses or eye drops can reduce a patient’s final prescription by half in the School of Optometry’s Myopia Control Clinic. That means thinner lenses and also a much lower risk of serious eye problems, including glaucoma and cataracts.