| 2010 Case #11 |  |
|
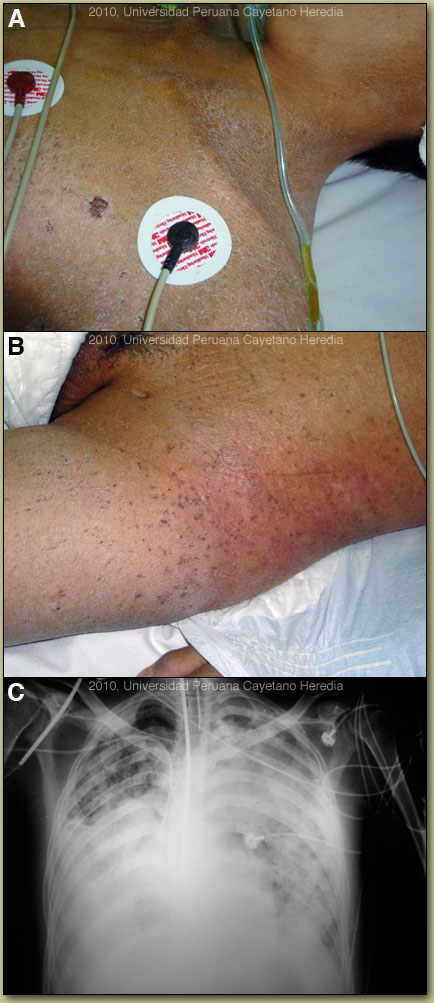
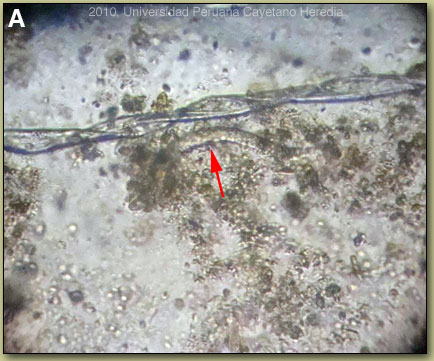
| Diagnosis: Disseminated hyperinfection with Strongyloides stercoralis and crusted (Norwegian) scabies secondary to HTLV-1infection. |
|
A number of conditions are associated with HTLV-1 infection; [see Lancet Infect Dis. 2007 Apr;7(4):266-81 for a detailed discussion]. This patient demonstrates at least two at the same time: crusted (Norwegian) scabies [see also Gorgas Case 2008-08] and strongyloides hyperinfection [see also Gorgas Case 2007-04]. Others include: tropical spastic paraparesis (TSP), also called HAM (HTLV-1 associated myelopathy) [see Gorgas Case 2002-08], infective dermatitis [see Gorgas Case 2004-07], adult T-cell leukemia/lymphoma (ATLL) [see Gorgas Case 2009-11], autoimmune disease including uveitis, Sjögrens, arthropathy, polymyositis, and thyroiditis. This patient did not have any neurological findings. More than 90% of individuals with HTLV-1 infection remain asymptomatic for life. Worldwide, approximately 0.3-4.0% develop TSP and 1-5% develop ATLL. Although the pathogenetic mechanisms of these two major complications likely differ, the low rates of each means that it is rare to see both sequelae in the same patient. In Perú 86% of patients who present with strongyloides hyperinfection are HTLV-1 positive [Am J Trop Med Hyg. 1999 Jan;60(1):146-9]. The prevalence of HTLV-1 in South America is generally underappreciated, normally being associated with Japanese and Caribbean populations. In Perú, the disease is highly endemic (2-3% seropositivity) in Andean areas of the country in Quechua populations who have had no contact with Japanese immigrants to the country. Other South American countries with significant rates of HTLV-1 include Brazil, Colombia, and Ecuador. We have seen over 800 infected families to date at the Tropical Medicine Institute in Lima. Strongyloides stercoralis has a complex life cycle. Three separate developmental stages play a role in the life cycle: adult worms, rhabditiform larvae, and filariform (infectious) larvae. Three alternative clinically important modes of replication exist. In the internal sexual cycle the adult female, resident in the small intestine, lays small numbers of eggs that hatch almost immediately. The resulting rhabditiform larvae are passed in the stool, mature in the soil to infective filariform larvae, and penetrate the skin of a new human host. After migration through the lung, the larvae crawl over the glottis, are swallowed and complete development in the jejeunum. In the free-living cycle, rhabditiform larvae may instead develop in the soil into sexually active adults, thus creating an environmental reservoir of adult worms. Each generation of this external sexual cycle includes a new cohort of infectious filariform larvae ready to re-enter the parasitic cycle in humans. Finally, in the autoinfective cycle, infectious filariform larvae develop from rhabditiform larvae while still in the intestine. Penetration of the colon or the anal skin by filariform larvae allows re-infection of the same host. The autoinfective cycles, which normally result in low-grade chronic infection in normal hosts, should be distinguished from the hyperinfection that occurs in immunocompromised hosts as a result of massive potentially fatal autoinfection with dissemination of larvae to numerous distant organs. Clinically, strongyloides infection is often asymptomatic. Purely intestinal infection may cause mild to severe abdominal symptoms. Adult females in the small intestinal mucosa may cause mild to severe inflammation. In autoinfective cycles, migrating larvae may cause serpiginous skin lesions (larva currens), especially around buttocks, or lung inflammation. Opportunistic behavior in immunocompromised hosts with hyperinfection may result in wide dissemination to extraintestinal organs, including the central nervous system, pulmonary hemorrhages, sepsis, and death. Inflammation and necrosis may occur in any organ and intestinal perforation may occur. Predisposing factors include malnutrition, corticosteroid therapy, malignancy, and HTLV-1 (but not HIV) infection. Our patient had two of these high risk factors HTLV-1 and then the erroneous treatment of the undiagnosed scabies skin lesion with systemic corticosteroids. Patients with strongyloides hyperinfection may have eosinophilia in the early stages of disseminated disease, but are most often eosinopenic by the time of clinical presentation. At the Tropical Medicine Institute in Lima approximately 70% of all cases of Norwegian scabies are associated with HTLV-1 infection and are, in the absence of any other factors, associated with immunosuppression. The lesions in this patient are highly characteristic [see also 2008-8] and frequently not recognized by inexperienced clinicians used only to classic scabies lesions in normal hosts. Norwegian scabies is not responsive to normal topical agents such as benzyl benzoate or permethrin. Patients are generally treated with with ivermectin 200 µg/kg bid for 2 days which is repeated 15 days later. The lesions responded dramatically to therapy. Despite broad-spectrum antibiotics, pressors, and mechanical ventilation this patient died a few days later. In addition, he received ivermectin through the nasogastric tube (200 µg/kg/d) for two consecutive days. |
 Discussion:
Discussion: