 |
Gorgas Case 2025-2 |
 |
|
The following patient was seen in the inpatient ward of the Cayetano Heredia Hospital (HCH) in Lima by the 2025 Gorgas Course participants.
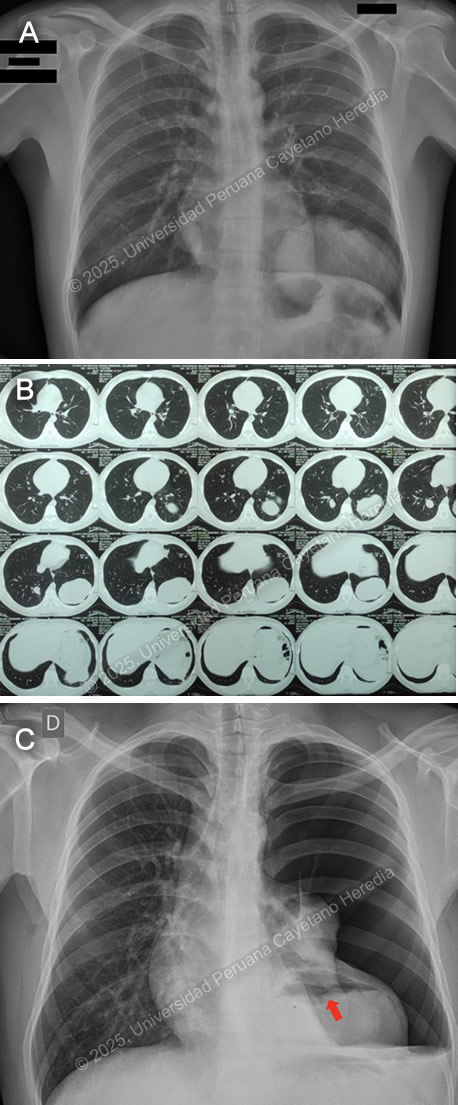 History: A 21-year-old male patient with no significant past medical history presented to the emergency department of HCH with a 5-month history of cough and chest pain. Five months before admission, he presented sporadic dry cough and occasional mild left pleuritic chest pain. He attended an occupational medicine consult where a routine chest X-ray was performed, revealing a radiopaque circular lesion in the left hemithorax (Image A), and a follow-up chest CT confirmed the presence of a 72x98x68mm left-sided pulmonary lesion and an additional right-sided 19x18x23mm hyperdense pulmonary lesion (Image B). Three weeks before admission his cough became productive with whitish sputum and was associated with shortness of breath on exertion. Ten days before admission, the pleuritic pain intensified and disrupted his sleep. Five days before admission, he presented a cough with blood-tinged sputum, generalized malaise, and subjective fever. Due to the persistence of symptoms, he traveled to Lima and attended the ED. Epidemiology: The patient was born and lives in Oyon, a city in northern Lima province at an altitude of 3600 meters above sea level. He lives in a rural area where he works as a mechanic and on his family’s farm, feeding, cleaning, and caring for sheep, cows, cats, and dogs. The dogs live inside the house and are fed on the dead sheep’s viscera. He has only traveled to rural areas nearby. He has no known TB contacts and no other family members have presented similar symptoms. Physical Examination on admission: BP was 100/60 mmHg, RR 28, HR 92, afebrile at 37°C with a Sp02 of 90% on room air. Breath sounds were abolished in the left hemithorax, and there was hyper resonance to percussion in the upper 2/3 and dullness in the lower 1/3 on the left hemithorax. The rest of the exam was normal. Laboratory: Hemoglobin was 17 g/dL (13-16 g/dL), WBC 17 100/uL (4-12 x 10^3/uL) with no bands, 51.6% neutrophils, 15.9% lymphocytes, 27% eosinophils (absolute count: 4 595, normal being less than 500/uL), 0% basophils, 4.9% monocytes. Platelets 391 000/uL. Urea 29 mg/dL, Creatinine 0.83 mg/dL, Sodium 142 mEq/L (135-145 mEq/L), Potassium 4.35 mEq/L (3.5-5.5 mEq/L), Chloride 107 mEq/L (96-106 mEq/L). Total bilirubin 0.53 g/dL with a direct of 0.32 g/dL. AST 14, ALT 24 IU/L (normal less than 40 IU/L). CRP 4.6 mg/dL (normal less than 0.3 mg/dL). PT 13.5 sec, PTT 29 sec with an INR of 1.15. HIV test was negative and VDRL was non-reactive. Imaging: A chest X-ray was performed on admission (Image C) followed by a normal abdominal ultrasound. UPCH Case Editors: Carlos Seas, Course Director / Mario Suito, Associate Coordinator |
|
 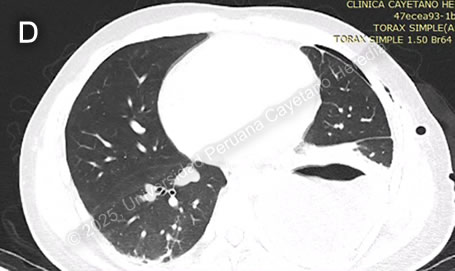 Discussion: The chest X-ray on admission revealed a left side hydropneumothorax with a ruptured cyst on the left lung base showing the “water lily” sign (Image C, red arrow), which was more evident on chest imaging after chest tube insertion (Image D). Western Blot for Echinococcus granulosus was positive for the 21kDa, 16kDa, and 8kDa antigens, confirming the diagnosis. PHD is the second most common manifestation of cystic echinococcosis (CE) representing 20-30% of all cases (1). CE has an annual incidence in endemic areas of 1 to 200 per 100,000 and a mortality rate of 2-4% (2), affecting countries from South America, Eastern Africa, Central Asia, and the Mediterranean (1). It is commonly found in impoverished rural areas. Most affected individuals have one or more risk factors, including living in unsanitary conditions, raising livestock such as sheep, herding or guarding dogs often near homes that are fed offal, and slaughtering livestock close to humans and dogs (3). Echinococcus granulosus eggs are released by the adult tapeworms residing in the definitive hosts (canids, mainly dogs) GI tract that are ingested by humans who act as accidental hosts. Eggs hatch in the intestine, releasing oncospheres that enter the portal circulation or lymphatic system, reaching the liver, where they develop into hydatid cysts (metacestode larvae). The hydatid cyst is composed of an acellular laminar layer and an internal germinal layer that produces a clear hydatid fluid, protoscoleces, and daughter cysts, and is surrounded by a third membrane, the adventitial layer (pericyst), formed by the host immune reaction to the cyst and fibrous tissue. Oncospheres are usually trapped in the hepatic parenchyma, but some may travel through the liver sinusoids and pass through the hepatic veins and inferior vena cava, reaching the right heart and landing in the lungs or pass the lungs and through the left side of the heart reach other organs. PHD has a variable clinical presentation depending on location, size, and cyst wall integrity. It can be discovered incidentally since it can be asymptomatic until rupture, fistula development, mass effect, or other complications occur (4). Some symptoms and signs associated with cysts include shortness of breath, coughing, hemoptysis, pneumonia, atelectasis, and the expectoration of a salty fluid (‘’vomica’’) when a cystobronchial fistula is formed (5). Diagnosis is based on radiological findings and complementary confirmatory serology. Serology includes sensitive screening tests like enzyme immunoassays (EIA) and specific confirmatory tests like immunoblot (Western blot). Screening tests have a sensitivity of 80-100% for liver cysts but only 50-56% for lung cysts. Therefore, a high false-negative incidence rate must be considered, particularly for cyst stages where the host has not been exposed to the parasitic antigen (CE1) or for biologically inactive cysts (CE4, CE5) (6). Surgery is the cornerstone of therapy for PHD. It should be pursued first in the co-existence of pulmonary and liver cysts because of the higher risk of rupture and cystobronchial fistulas of the former (5). Treatment with albendazole 10-15 mg/kg divided into two doses can be started 1-3 days before surgery and continued 3 months postoperatively. In cases where the cyst contents spill into the pleural space either spontaneously or iatrogenically, as in our patient’s case, it is essential to begin antiparasitic treatment promptly alongside surgical intervention and thorough irrigation of the pleural space with isotonic saline to prevent secondary CE (5). Primary antiparasitic treatment without surgical involvement is usually avoided because of the risk of treatment-induced cyst membrane detachment, rupture, and opening of cystobronchial fistulas, except for small lung cysts less than 5 cm (7). References |