 |
Gorgas Case 2025-8 |
 |
|
The following patient was seen on the inpatient ward of Cayetano Heredia Hospital in Lima by the 2025 Gorgas Course participants.
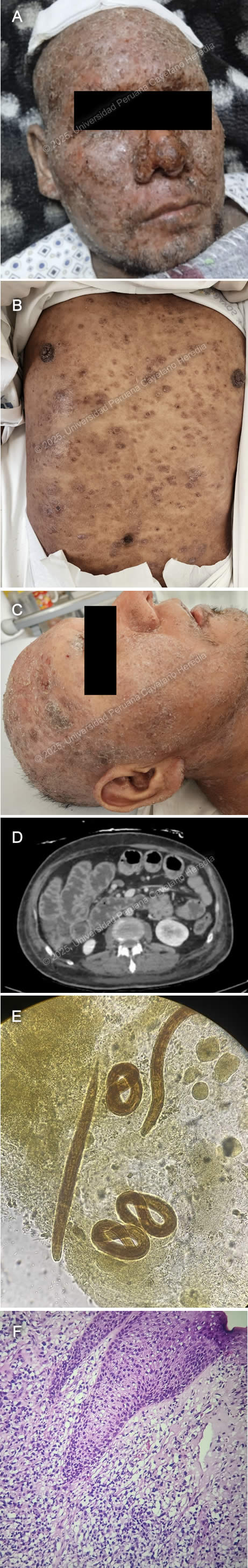 History: A 39-year-old male patient presented with an eight-year history of progressive, disseminated, pruritic cutaneous lesions and a five-day history of diarrhea, nausea, and vomiting. Eight years before admission, he developed erythematous, painful, and pruritic plaques on the lower extremities and back. Over the following year, the lesions progressed to involve the entire body, including the scalp and face, and were associated with patchy hair loss. He sought care at multiple primary healthcare centers, where topical clobetasol was prescribed with partial improvement. Symptoms persisted and two weeks before admission, he developed fever, chills, and generalized malaise, with worsening of his skin lesions, characterized by increased pain and pruritus (Image A). He was hospitalized at a regional hospital for seven days and discharged with amoxicillin-clavulanic acid and oral prednisone (50 mg/24h), with partial improvement and a presumptive diagnosis of pemphigus vulgaris. Five days before admission, he developed diarrhea, nausea, and vomiting (4-5 episodes per day), leading to appetite loss and an inability to tolerate solid food. Symptoms persisted, and on the day of admission, he experienced severe generalized weakness, prompting his family to bring him to the emergency room. Epidemiology: The patient is the eldest of nine siblings, all reportedly healthy. His father had a history of similar pruritic skin lesions, though less severe, and died of cirrhosis. His wife has five siblings, all apparently healthy. Socially, he has worked in construction and agriculture in his youth and as a bus driver since the age of 27. He has lived in Ayacucho (until 24 years old), Arequipa (until 36 years old), and then returned to Ayacucho, residing in a two-room adobe house with sewage, water, and electricity, shared with four people. He raises chickens at home. In June 2024, he traveled to Pichari, La Convención (a jungle region). He has no known history of tuberculosis exposure. Physical Examination on admission: On admission, the patient was in poor general condition with vital signs showing tachycardia (HR 118 bpm), normal respiratory rate (RR 16), afebrile (36°C), blood pressure of 118/98 mmHg, and oxygen saturation of 98% on room air (FiO2 0.21). Skin examination revealed warm, dry, disseminated erythematous papules and plaques with moderate scaling and erosions (Image B) and nodular lesions on the scalp (Image C). The patient had mild bilateral lower extremity edema and diffuse lymph node enlargement. Abdominal examination showed epigastric tenderness and dullness over Traube’s space. Neurologically, he was alert and oriented (GCS 15/15) with no meningeal signs or focal deficits. The cardiovascular, respiratory, genitourinary, and musculoskeletal examinations were unremarkable. Laboratory: On admission the patient exhibited a hemoglobin level of 12.3 g/dL, leukocytosis with WBC 10.1 ×10⁹/L, and neutrophil predominance (63.3%, absolute count 6.39 ×10⁹/L). Eosinophilia was noted (13.9%, absolute count 1.4 ×10⁹/L), along with thrombocytosis (561,000/μL, previously 626,000/μL). Biochemistry showed hyponatremia (Na⁺ 123 mmol/L), hypochloremia (Cl⁻ 85 mmol/L), and hypoalbuminemia (2.2 g/dL), with total protein at 4.7 g/dL. Kidney function was stable (creatinine 0.52 mg/dL, urea not reported on DOA). Liver function tests were within normal limits (AST 20 U/L, ALT 13 U/L, total bilirubin 0.6 mg/dL). Coagulation parameters showed PT 13.3 sec, PTT 36 sec, and INR 1.17. Inflammatory markers included an elevated CRP (37 mg/L). Lipase was mildly elevated (104.6 U/L), while amylase (49 U/L) and CPK (23 U/L) were within normal ranges. Serological tests were positive for HTLV-1 and anti-HBc, while HBsAg, HIV and hepatitis C tests were negative. Imaging: Abdominal CT revealed diffuse small and large intestine wall thickening with air-fluid levels with no mechanical obstruction concerning for ileus (Image D). Microbiology: Ova and parasite (O&P) from gastric aspirate, stool and sputum revealed the following structures (Image E). Histopathology: Skin biopsy from the lesions revealed a lymphocytic infiltrate of the dermis invading the epidermis with variable sized lymphocytes with irregular-shaped nuclei (Image F) that stained positive for CD3 and CD4 on immunohistochemistry. UPCH Case Editors: Carlos Seas, Course Director / Mario Suito, Associate Coordinator |
|
Discussion: Skin histopathology revealed findings compatible with ATLL. O&P revealed both filariform and rhabditiform larvae including from the sputum, confirming the diagnosis of hyperinfection syndrome. ATLL is a malignant complication of the often-asymptomatic HTLV-1 infection (1), and its incidence varies according to the endemicity of HTLV-1, the most common places being Southwestern Japan, the Caribbean basin, Western Africa, Iran and Peru (2). It usually develops in chronically infected patients between 60-70 years of age (3) and both treatment and prognosis depend on the clinical subtype of ATLL (4). The most common presentation (60%) with the worst prognosis (8-month median survival) is the acute form typically presenting with fever, weight loss, generalized lymphadenopathy, hepatosplenomegaly and lymphocytosis with atypical lymphocytes, some presenting pathognomonic flower-cell appearance. Around 50% of patients also have hypercalcemia. Skin involvement occurs in 25% of patients with tumor-like lesions being the most frequent manifestation (39%), followed by plaques (27%), papules (19%), patches (7%), purpuric lesions (4%) and erythroderma (4%) (5). Prognosis is also influenced by the type of cutaneous lesions, with the nodular/tumoral lesions presenting the worst prognosis (6). Lymphomatous ATLL is the second most common subtype (20%) with similar symptoms but without lymphocytosis. Chronic ATLL occurs in 10% of cases and usually presents with months-to-years of cutaneous lesions, lymphadenopathy and lymphocytosis. Smoldering ATLL is the least frequent subtype (< 10%) with patients usually being asymptomatic with < 5% of circulating neoplastic cells. Diagnosis is made with the identification of characteristic malignant cells on peripheral blood smear, bone marrow or histopathology of cutaneous lesions, and the confirmation of HTLV-1 infection. ATLL malignant lymphocytes are characterized by hyperlobated irregular nuclei with flower-cell appearance with the characteristic CD3+, CD4+, CD25+, CD7-, CD8- immunophenotype. In selected instances HTLV-1 proviral clonality of malignant cells can be demonstrated with PCR based methods (7). Remarkably, Strongyloides stercoralis (Ss) coinfected patients tend to develop ATLL earlier (39 vs 60-70 years of age) (8). The leading hypothesis for this is 1) chronic antigenic stimulation of infected cells leads to accelerated oncogenic mutation accumulation 2) increased proportion of T regulatory lymphocytes caused by Ss downregulates the immune response against malignant lymphocytes and 3) microbial translocation from the GI tract induced chronic inflammation. Ss is geohelminth that shares many of the tropical and subtropical regions were HTLV-1 is endemic, including Peru and affects approx. 50-100 million individuals worldwide (9, 10). Filariform larvae penetrate areas of intact skin, migrate through the circulatory system until reaching the lungs where they access the respiratory tract, climb up until reaching the GI tract where the adult females reside and multiply. Half of those infected remain asymptomatic. The most common symptoms are usually mild gastrointestinal symptoms and eosinophilia. Immunosuppressed patients, particularly those coinfected with HTLV-1 or receiving corticosteroids can develop either strongyloides hyperinfection syndrome with involvement of the GI, respiratory and cutaneous systems or disseminated strongyloidiasis with extra-Loeffler cycle organ involvement (11). The bidirectional relationship between worsening clinical manifestations and outcomes in HTLV-1 and Ss-coinfected patients highlights the neglected nature of these infectious organisms and the need for further research into their combined pathophysiology. Our patient was started on subcutaneous ivermectin, and intravenous antibiotics. The subcutaneous (SC) route for administering ivermectin has been used in patients who cannot tolerate the oral route with mixed results (12). The SC preparation is approved for veterinary use only. Therefore, consent was obtained from the patient. No actual dosing and regimen recommendations exist. He is receiving 200 ugr/kg per day. References |