 |
Gorgas Case 2025-3 |
 |
|
The following patient was seen in the inpatient ward of Cayetano Heredia Hospital (HCH) in Lima by the 2025 Gorgas Course participants.
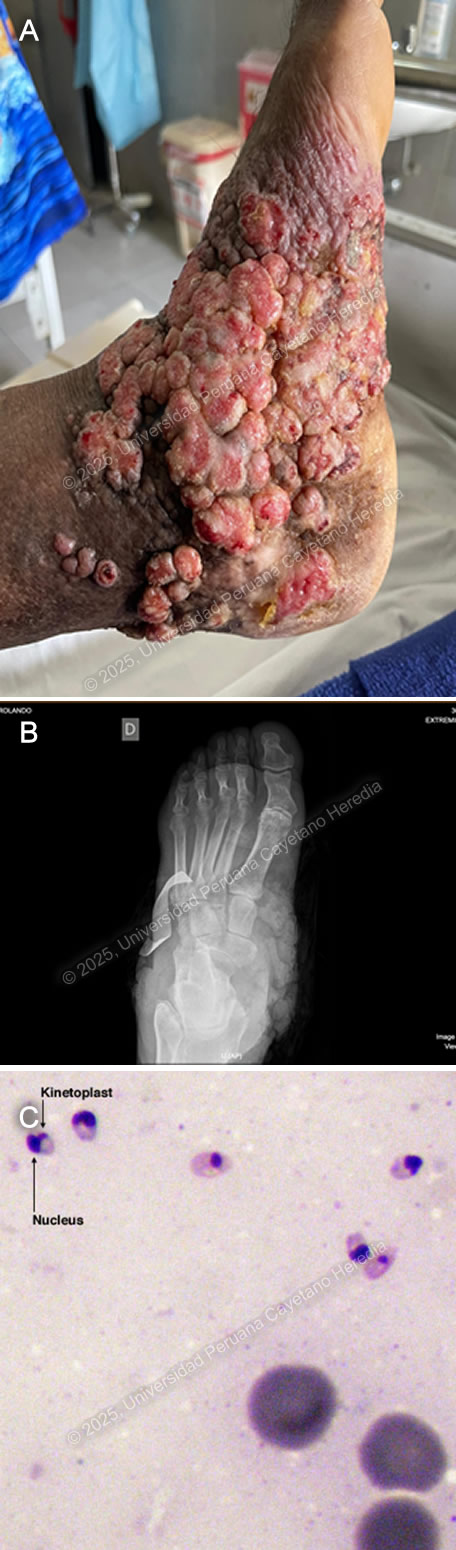 History: A 70-year-old male patient with no significant past medical history presented to the outpatient clinic at HCH with a six-year history of expanding, painless skin lesions on his left foot. Six years before admission, he developed a painless, non-pruritic, 1 x 1.5 cm non-healing papule that became ulcerated on the dorsum of his left foot. At that time, he sought care at a local health center, where he received three intralesional pentavalent antimonial injections over three consecutive days, with partial clinical improvement. Five years before admission, the lesion increased in size to 5 x 5 cm and was accompanied by localized swelling. The lesion continued to expand, and four months before admission, it became moderately painful, impairing his daily activities. Due to persistent symptoms, he attended the dermatology clinic and was admitted for further workup and management. Epidemiology: The patient was born and resides in La Merced, a city located in the high jungle of Peru, where he works in agriculture cultivating coffee and fruits. He frequently walks barefoot in the jungle soil and experiences numerous mosquito bites. He denies any travel outside his place of birth. He has no known tuberculosis (TB) contacts but reports that multiple neighbors throughout his lifetime have presented with similar non-healing cutaneous ulcers. Physical Examination on admission: BP: 110/80 mmHg, HR: 70 bpm, RR: 20 breaths per minute, T: 36.5°C, SpO2: 98% on room air. The skin examination revealed multiple tumor-like, verrucous, confluent hyperkeratotic plaques with irregular and poorly defined borders in the middle and lateral malleolar regions and on the heel of the left foot, with hyperpigmentation and scaling (Image A). The rest of the examination was normal. Laboratory: Hemoglobin: 9.4 g/dL (MCV 92 fL, MCHC 33 g/dL), Hematocrit: 29%, WBC: 4,400/µL (0% bands, 65.1% neutrophils, 8.7% eosinophils, 0.5% basophils, 11.3% monocytes, 14.4% lymphocytes), Platelets: 433,000/µL, Creatinine: 0.81 mg/dL, Sodium: 140 mEq/L, Potassium: 4.9 mEq/L, Chloride: 104 mEq/L. Serology for HIV, HTLV-1, RPR, and Hepatitis B and C were all negative. A foot X-ray showed no bone involvement (Image B). A Giemsa-stained scraping from the border of a lesion was performed (Image C; reference image, original of low quality). UPCH Case Editors: Carlos Seas, Course Director / Mario Suito, Associate Coordinator |
|
 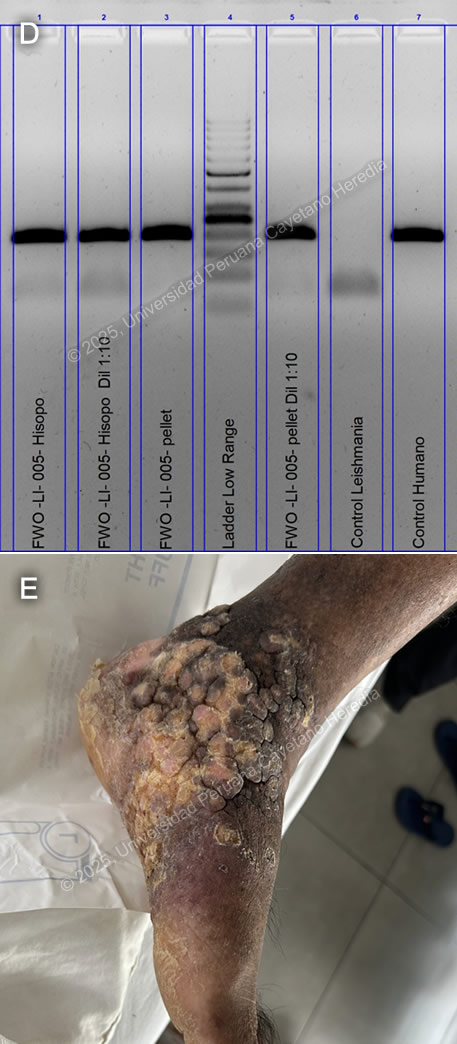 Discussion: GeneXpert ultra and acid-fast bacilli (AFB) staining from the tissue biopsy were negative, as was the TB liquid media (MGIT) culture. KOH and fungal cultures were also negative. The Montenegro intradermal hypersensitivity test was positive (30 x 35 mm). A skin punch biopsy revealed parakeratosis and acanthosis associated with a lymphohistiocytic inflammatory infiltrate and epithelioid granulomas; however, no definitive amastigotes were seen. A kinetoplast DNA PCR (kDNA PCR) for Leishmania sp. was positive, confirming the diagnosis (Image D). Finally, the patient presented a remarkably positive therapeutic response after treatment with Amphotericin B, showing marked improvement of the lesion (Image E). Leishmaniasis is endemic in 88 countries, including Brazil, French Guiana, Colombia, Ecuador, Bolivia, and Peru (1). The most common manifestation is cutaneous leishmaniasis (CL), which affects 10 million people worldwide. Affected patients typically live in or have a history of travel to endemic areas. In Peru, over 7,000 cases are reported annually (2,3). CL in Peru is a zoonosis caused by Leishmania species, almost exclusively from the subgenus Viannia, particularly L. peruviana, L. braziliensis, L. guyanensis, and L. panamensis. Humans become infected when the sandfly vector (Lutzomyia sp.) inoculates promastigotes into the host’s bloodstream while taking a blood meal. These promastigotes are phagocytized by macrophages, where they develop into amastigotes that multiply until they are released and infect other cells. CL usually develops 2-8 weeks after inoculation. The most common presentations include the localized-ulcerative form, the nodular or sporotrichoid form, and the diffuse and disseminated cutaneous forms. Less common manifestations include plaque, psoriasiform, and verrucous leishmaniasis, which represent 2-5% of all CL cases (4). Verrucous leishmaniasis is a rare subtype of the already infrequent atypical cutaneous leishmaniasis subgroup. It is classically described as hyperkeratotic plaques covered by thick, adherent crusts. Diagnosis relies on identifying high-risk patients based on compatible clinical manifestations and the appropriate epidemiological exposure, followed by specific parasitological diagnostic tests. Most confirmatory tests require invasive tissue biopsies, where 1) direct microscopy with commonly used Giemsa stain reveals amastigotes, 2) culture from the lesion demonstrates promastigotes, and/or 3) PCR amplification detects genomic DNA from Leishmania sp. Serology has no role in the diagnosis of leishmaniasis. A positive Montenegro skin test demonstrates a delayed-type hypersensitivity reaction to Leishmania antigens but cannot differentiate between previous exposure and active disease. Speciation impacts treatment decisions regarding initiation, regimen selection, duration, and dosage. It can be performed using isoenzyme analysis requiring cultured promastigotes or molecular methods like PCR from a tissue sample (5). New World cutaneous leishmaniasis may be treated topically with paromomycin and gentamycin, intralesional injections of pentavalent antimonials, or physical methods such as thermal and cryotherapy (6). For patients with extensive (>5 cm) or multiple (≥ 5) lesions, immunosuppression, or species associated with mucosal disease (L. braziliensis, L. guyanensis), systemic treatment is recommended. Systemic options include intravenous pentavalent antimonials (SbV) (sodium stibogluconate, meglumine antimoniate) and Amphotericin B, as well as oral Miltefosine (7). Although no controlled randomized trials have demonstrated a significant statistical difference between amphotericin B and SbV, extensive cutaneous disease, such as in this case, is typically treated with Amphotericin B (8). References |