 Dr. Laura James, University of Arkansas Medical Center (UAMS) Associate Vice Chancellor of Clinical and Translational Research and Translational Research Institute Director, shared her team's success story in leveraging NIH Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) grants to fund their research and commercialize a new diagnostic test for acetaminophen toxicity.
Dr. Laura James, University of Arkansas Medical Center (UAMS) Associate Vice Chancellor of Clinical and Translational Research and Translational Research Institute Director, shared her team's success story in leveraging NIH Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) grants to fund their research and commercialize a new diagnostic test for acetaminophen toxicity.
What are SBIR/STTR Grants?
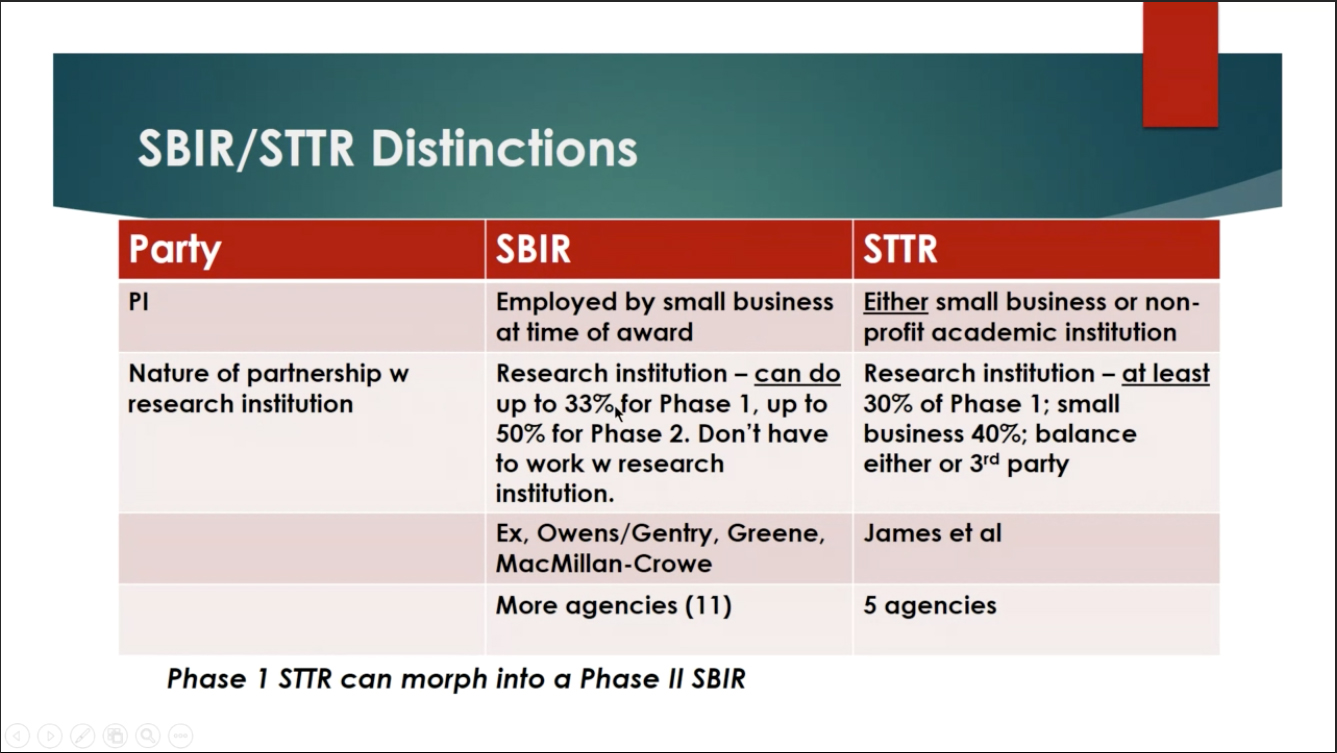
 James reviewed the purpose of these NIH funding mechanisms, which represent one of the largest sources of early-stage technology financing in the country. The goal of SBIRs is to strengthen the role of innovative small business concerns in federally funded research and development (R&D). STTRs facilitate technology transfer developed by a research institution through the entrepreneurship of a small business concern.
James reviewed the purpose of these NIH funding mechanisms, which represent one of the largest sources of early-stage technology financing in the country. The goal of SBIRs is to strengthen the role of innovative small business concerns in federally funded research and development (R&D). STTRs facilitate technology transfer developed by a research institution through the entrepreneurship of a small business concern.
Phase I and II, James explained, are when you build and test your prototype via a clinical trial or some similar study design. Small businesses that have performed Phase I-type research through other funding sources may submit a Direct-to-Phase II SBIR application. Phase IIB, which used to be called Phase III, is the final phase, when an investigator moves a discovery validated by earlier phases into commercialization.
“The program continually builds upon itself. So if you apply for a Phase I grant, which is entry level, and you’re successful, then you are eligible to go on to Phase II, which is a bigger award for more years of funding, plus NIH mentoring that we found to be very helpful,” James said. She recommended investigators explore an interactive SBIR/STTR infographic that provides a tutorial for applicants taking them from “a good idea” through the different funding pathways and corresponding levels of support each phase provides for R&D or commercialization.
Benefits of SBIR/STTR Funding
Since James first heard of the SBIR/STTR grants in 2007, the program has grown and is now NIH-wide, with every Institute offering some funding via one or both of these mechanisms. She noted they represent a growing share of NIH Institute budgets. They are attractive for many reasons, including “they are generally less competitive, as fewer people know about them.” Other benefits include:
- Funding is stable, predictable, and not a loan
- Funding lines are generally higher than an R01
- Capital is non-dilutive (no need to give up an equity stake or share ownership as with private sources of capital funding)
- Small businesses and research organizations retain intellectual property rights
- SBIR and STTR grants confer eligibility to use other NIH support programs and resources
- NIH’s rigorous peer-review process for these grants provides recognition, validation, and visibility to early-stage biomedical companies
- The prestige associated with these awards can help attract more funding or other support (e.g., venture capital, strategic partners)
Of critical importance to James, who did not wish to leave academia, the STTR grants she received allowed her to maintain her academic career and even helped offset her academic salary. Indeed, the STTR grant requires that applicants have a partnership with an academic institution.
Acetaminophen Toxicity Diagnostics (ATD) LLC

James began her career as pediatrician and became involved in clinical trials, eventually joining a multidisciplinary team that wanted to improve the ability to diagnose liver injury (formation of adducts due to acetaminophen poisoning) at the point of care.
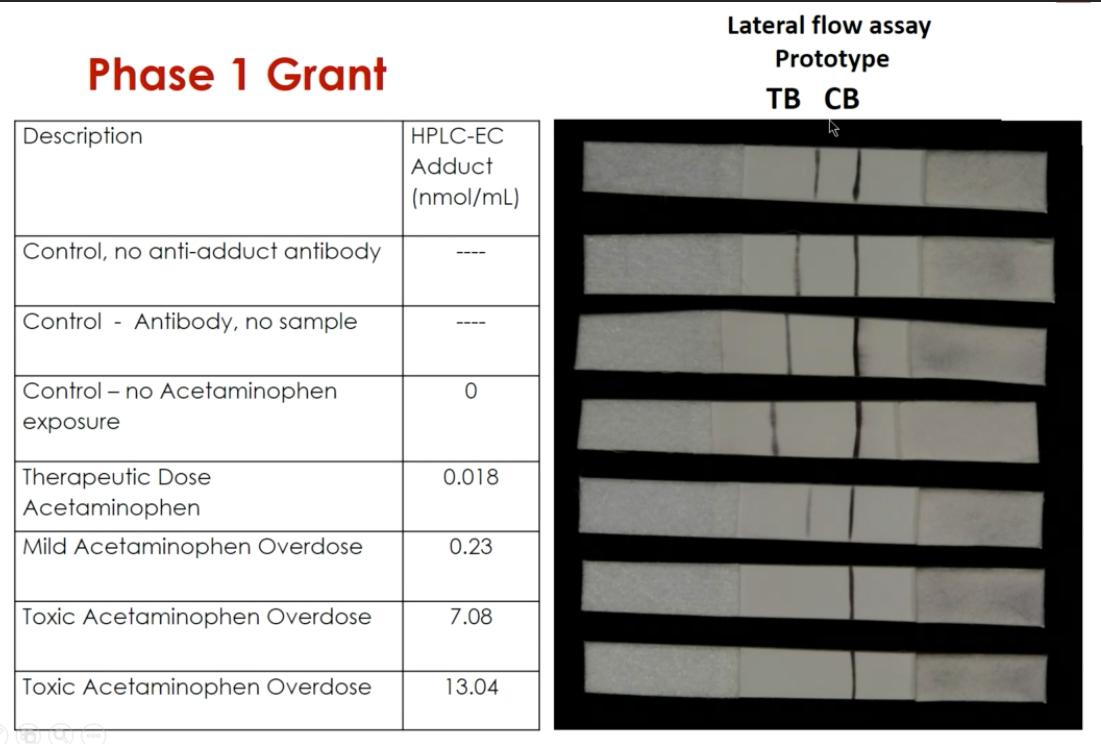
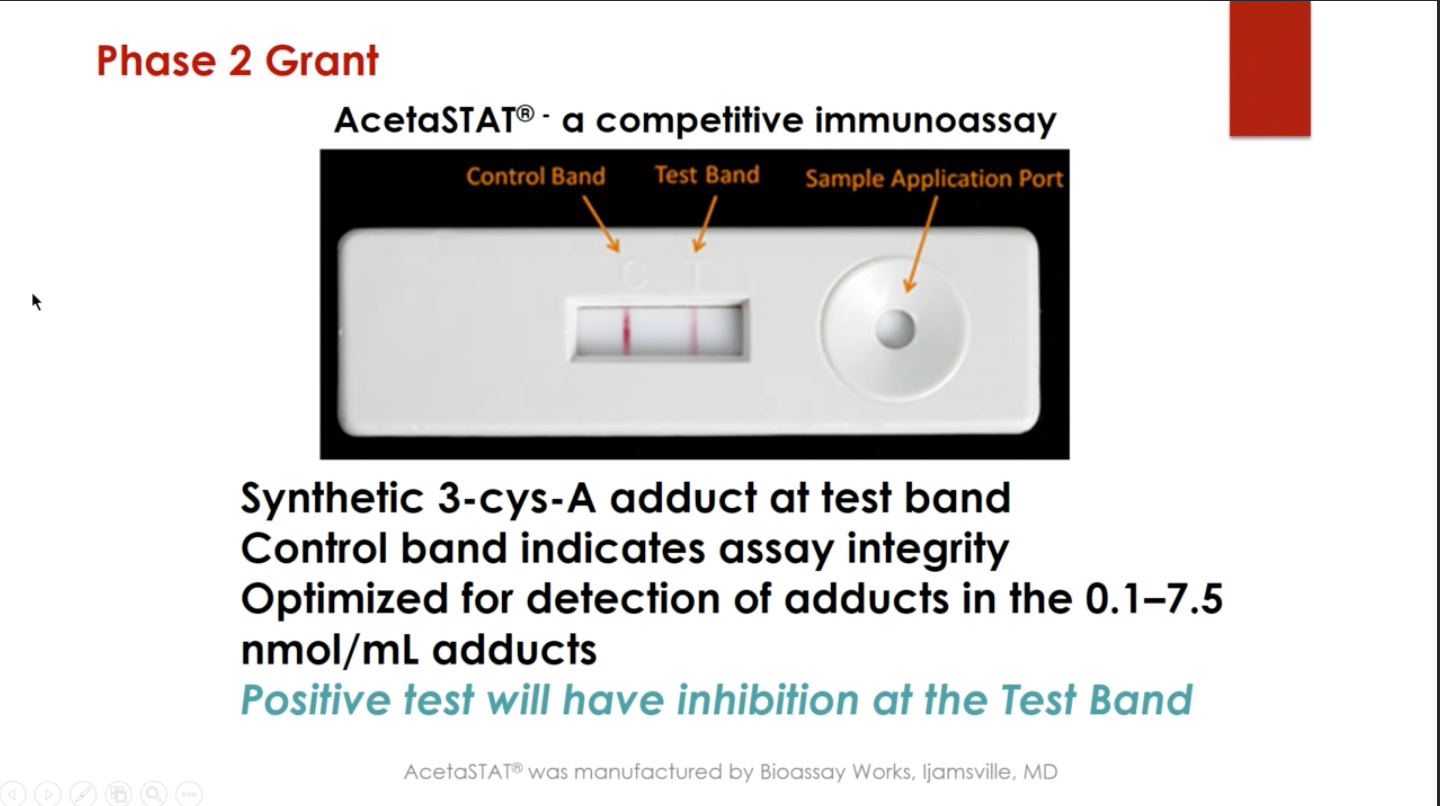
The team members formed a company in 2006 and received their first grant in 2007 to develop an assay that could detect acetaminophen protein adducts. They applied for a supplement in 2010 to develop instrumentation (a dipstick for the assay, similar to a urine pregnancy test), followed by grants to fund clinical testing of their product, AcetaSTAT®.
James emphasized that ATD “did not happen overnight, but grew out of a long-standing collaboration” and built with lots of “local support.” This was one of the keys to success, in addition to defining the clinical problem in a way that clearly elucidated “where is the pain?” and putting together a well-rounded team.
To hear James’ entire talk, which traces the journey of her team’s discovery, product development, and company in relation to the support they received, including collaborators and STTR funding, click here.
Current NCATS SBIR/STTR Opportunities
The National Center for Advancing Clinical and Translational Science (NCATS) bills its SBIR and STTR programs as “engines of innovation for developing and commercializing tools, technologies and intervention platforms to support the creation of new therapeutics and diagnostics.”
NCATS recently released two new SBIR/STTR funding opportunities through the 2018 Omnibus Solicitation. Representatives of small businesses and research organizations focused on commercializing innovative medical technologies are encouraged to submit applications to PA-18-574 and PA-18-575 by Jan. 7, 2019.
NCATS encourages applications that address research areas relevant to any stage of translation, from target validation through pre-clinical and clinical evaluation to intervention implementation and dissemination, including:
- Drug Discovery and Development
- Biomedical, Clinical and Health Research Informatics
- Clinical, Dissemination and Implementation Research
An updated SBIR/STTR Application Guide with additional instructions for the newly reinstated SBIR Direct Phase II application preparation and submission will be posted by Nov. 26, 2018.