The CCTS is committed to collaborative innovation and pursues several partnerships that extend across the Hub, throughout the region, between CTSA Hubs, with the National CTSA Consortium, and beyond. These partnerships leverage the unique and complementary research strengths and expertise of multiple institutions to accelerate clinical and translational discovery in clinical and bioinformatics, training, pediatric research, maternal/fetal medicine, and exercise. They also result in operational synergies and sharing of best practices. We invite you to explore our list of collaborators.
-
Academic Drug Discovery & Device Development Program
Academic Drug Discovery & Device Development Program
The CCTS Hub works with regional partners to facilitate and promote unique research opportunities. Through the Academic Drug Discovery and Device Development Program (AD4), CCTS investigators can access the leading-edge, high through-put screening and drug development capacity at Southern Research (SR) to assay new molecular targets, develop effective screens for novel targets, accelerate potential therapies through the development pipeline, and find new applications for existing clinically tested drugs (“re-purposing”).
The CCTS has more recently launched a device development initiative to support the identification and prototyping of medical device applications.
One such project, led by CCTS Partners Drs. Silas Leavesley and Tom Rich from the University of South Alabama (USA),
explores the use of spectral imaging to improve the detection of cancerous lesions in the colon. This CCTS video provides an in-depth
look at this exciting collaboration.Both drug and device programs are supported by multidisciplinary project development teams that provide expertise tailored to the study aims, including medicinal chemistry, high-throughput assay development, engineering, clinical application, and commercialization. The team meets quarterly to guide the project's progress and to troubleshoot obstacles. These discussions are conducted under a master Confidentiality Disclosure Agreement. In some cases, CCTS joins partnering institutions to financially enable assay/prototype development following a rigorous application and vetting process.
As projects mature, the collaborative development and commercialization of a drug or a device becomes the basis of a business agreement between Southern Research (or another company) and the investigator’s institution. Intellectual Property (IP) for drug or device efforts is negotiated between institutions that have a significant inventive contribution to the project. Regardless, such projects may continue to benefit from CCTS-organized consultation and project development.
AD4 represents an expansion of CCTS involvement in the Alabama Drug Discovery Alliance and AIMTech.
To hear about these and other funding opportunities, subscribe to our CCTS Digest.
-
Child Health Research Acceleration through Multisite Planning
Child Health Research Acceleration through Multisite Planning
Building on this extensive foundation of expertise and capacity, the CCTS Network is an active member of the CTSA-driven Child Health Research Acceleration through Multisite Planning (CHAMP). CHAMP has assembled expertise and resources across multiple CTSA hubs to support pediatric investigators at the earliest phases of multisite clinical research. The initiative has created a portal to provide nascent research groups with the tools they need to perform clinical and translational research including recruitment strategies, data management and informatics tools, team building models, IRB guidance and contracting processes and facilitated interaction with national research networks. The CCTS Hub joins CHAMP member institutions including Cincinnati Children’s – University of Cincinnati, University of Massachusetts, Medical College of Wisconsin and New York University in support of research projects identified through competitive review.
-
National Exercise Clinical Trials Network
National Exercise Clinical Trials Network
With leadership from Dr. Marcas Bamman, the National Exercise Clinical Trials Network (NExTNet) has catalogued resources, expertise and equipment inventories across a 57-member consortium of CTSA Hubs and other academic medical centers for testing cardiopulmonary function, neuromuscular function, body composition, and metabolites; resistance training; and endurance or interval training. Among these resources is the Heritage (HEalth, RIsk factors, exercise Training And GEnetics) Family Study, led by Dr. Claude Bouchard of Pennington Biomedical Research Center, representing a major cohort used to document the impact of genetics on cardiovascular, metabolic and hormonal response to exercise. NExTNet has also developed the infrastructure for data sharing for completed exercise studies to support multi-site exercise clinical trials to address these scientific opportunities in a disease-specific or population-specific manner.
-
National Perinatal Research Consortium
National Perinatal Research Consortium
Through its support of clinical research expertise, the CCTS extends the capacity of major network initiatives that build on the strengths of the Partner Network and that leverage the opportunities throughout the country in pediatric research. Under the leadership of Drs. Joseph Biggio and Alan Tita, the CCTS’ National Perinatal Research Consortium (NPRC) is working with four other CTSA hubs (Columbia University, and the Universities of North Carolina, Utah, Texas Medical Branch in Galveston) to create a CTSA-supported Perinatal Research Network Consortium for clinical studies not within the purview of the current NICHD-funded Maternal Fetal Medicine Network, which we also support. The NPRC is committed to building a robust research infrastructure providing the highest and most reliable recruitment, retention and data standards to most effectively facilitate perinatal clinical, translational and comparative effectiveness research to improve health outcomes for women, their children, and their families. The group is currently developing the Registry of Rare Events in Obstetrics (RARE) that aims to compile cohorts of women with relatively rare conditions in pregnancy and compare outcomes across centers. -
Neonatal Kidney Collaborative
Neonatal Kidney Collaborative
The Neonatal Kidney Collaborative (NKC) is an alliance of neonatologists and pediatric nephrologists dedicated to improving outcomes in neonates at risk for acute kidney injury. NKC's mission is to improve the understanding and outcomes associated with kidney health in newborns globally. Its vision is to improve the lives of babies worldwide by performing high-quality clinical research, providing meaningful education to clinicians, enabling providers with safe/effective therapies, and advocating for neonates at risk for kidney disease.
Spearheaded by Dr. David Askenazi, the group has developed a robust infrastructure to support collaboration among an international network of neonatologists, pediatric nephrologists, obstetricians and pediatricians. The NKC is currently engaged in a variety of multi-center studies supported by this multidisciplinary approach.
Dr. Askenazi also works closely with Dr. Namasivayam Ambalavanan, MD, Director of the Translational Research in Normal & Disordered Development (TReNDD) Program, a collaborative group of investigators applying state of the art biological approaches to understanding human disorders of organogenesis.

-
Patient-Reported Outcomes Measurement Information System
Patient-Reported Outcomes Measurement Information System
Patient-reported outcomes (PROs) reflect the experience of health and healthcare as reported directly by the patient. There is increasing evidence that capturing PROs will be an essential component of quality measurement, quality improvement, and patient engagement in care and research. The Patient-Reported Outcomes Measurement Information System (PROMIS) toolset is a PRO survey system that utilizes computer adaptive testing to provide precise measurements with a minimum number of questions, often shortening conventional PRO surveys by 10-fold or more.
As part of this Collaborative Innovation effort, the CCTS joins teams at Northwestern, University of Chicago, University of Illinois at Chicago, Case Western, and the University of Utah to develop, implement and evaluate a seamless integration of the PROMIS toolset into the Cerner electronic health record utilizing the SMART-on-FHIR standard.

-
SMART IRB
SMART IRB
The CCTS promotes IRB reliance through NCATS' Streamlined, Multisite, Accelerated Reliance for Trials (SMART) IRB to support the protection of human subjects in multisite research projects. Use of SMART IRB not only reduces time-to-activation for clinical trials and studies based on two or more sites, but also facilitates negotiation of multisite clinical trial contracts. All CCTS Partners that perform interventional human subjects research have formally agreed to use IRB reliance and are joining SMART IRB.
SMART IRB also offers educational materials, tools, and checklists to support the review and conduct of multisite research, including streamlined standard operating procedures.
-
Southeast SHRINE Consortium
Southeast SHRINE Consortium
As part of the Southeastern SHRINE Network, the CCTS is collaborating with CTSA Hubs including Emory, University of Arkansas, University of Kentucky and Medical University of South Carolina, to federate available data at each institution for assessment of clinical trial feasibility, participant recruitment and comparative effectiveness research.
All partnering institutions bring strong experience in the deployment, use and extension of i2b2, in federated query infrastructure, and in participation in the development of PCORnet-style data sharing. Together, the group has developed and is maintaining i2b2 and SHRINE instances with a common SHRINE ontology, focused on multiple testbed areas of care and has established a shared governance model to access from the data network.
-
Trial Innovation Network
Trial Innovation Network
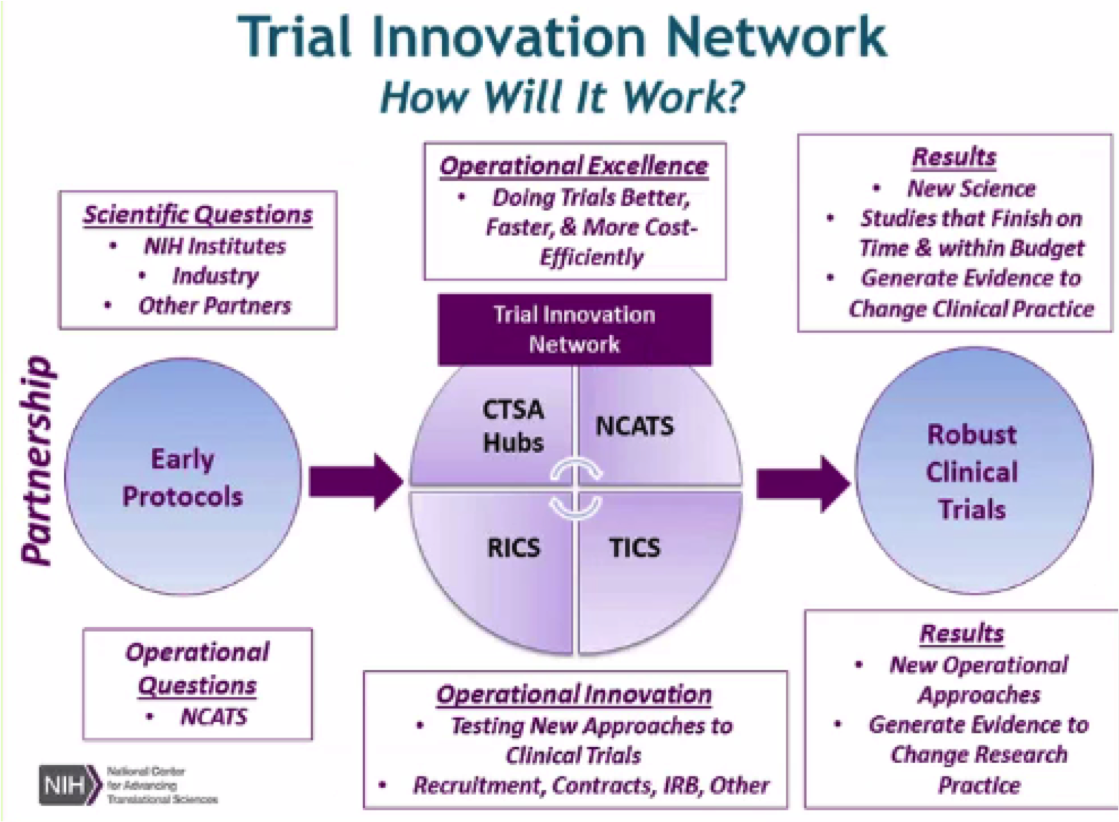 The NCATS Trial Innovation Network (TIN) seeks to address roadblocks in clinical trials and to accelerate the translation of novel interventions into life-saving therapies. The TIN focuses on innovations in two key areas traditionally associated with creating administrative burdens for multisite studies: trial initiation and patient recruitment. It comprises three key organizational partners:
The NCATS Trial Innovation Network (TIN) seeks to address roadblocks in clinical trials and to accelerate the translation of novel interventions into life-saving therapies. The TIN focuses on innovations in two key areas traditionally associated with creating administrative burdens for multisite studies: trial initiation and patient recruitment. It comprises three key organizational partners:-
Trial Innovation Centers (TICs)
-
Recruitment Innovation Center (RIC)
-
CTSA Program Hubs
The CCTS Hub has established a TIN Liaison team, which is responsible for coordinating with the national Trial Innovation Network. The TIN Liaison team helps provide reliable study activation, recruitment, retention, and data standards to most effectively facilitate clinical, translational, and comparative effectiveness multisite research focused on improving health outcomes. The team meets weekly and coordinates activities among other multisite study stakeholders, including the IRB, the Office of Sponsored Programs, CCTS Informatics and the Clinical Trials Administrative Office.
The team also interfaces with clinical and translational investigators through the CCTS Research Commons and helps connect the TIN to the capacities of our SHARe platform and so expand the reach of studies to the special populations in our region, which is diverse in ancestry, gender, geographic residence (rural and urban), socioeconomic status and disease burden.
Dr. Jason Nichols, Senior Associate Vice President for Research, oversees Multisite Study Support (MSSS) on behalf of the CCTS, serving as administrative director of both SHARe and the TIN Hub liaison team. Building on his experience with industry-sponsored clinical trials, Nichols assists in the development of the industry-based research portfolio at the CCTS Hub. He works closely with CCTS investigators across the Partner Network to develop multisite projects and to expedite contract and human subjects review to enable study activation.
Trial Innovation Network Hub Liaison Team
ROLE TEAM MEMBER Administrative Director Jason J. Nichols, OD MPH PhD
Senior Associate Vice President for Research (UAB)Medical Director Robert P. Kimberly, MD
Senior Associate Dean for Research Clinical & Translational Research, CCTS Director and Associate VP for Medicine & Biomedical ResearchProgram Co-Directors Frannie Horn, JD EdS, Program Director, CCTS / SHARe / TIN Hub
Jennifer Croker, PhD, CCTS Senior Administrative DirectorTraining, Contracting, Regulatory & Recruitment Meredith Fitz-Gerald, MSN
Director, CCTS Clinical Research Support Program -
-
TriNetX
TriNetX
One of the most common reasons a clinical trial fails is poor recruitment—it can be difficult to find enough patients who meet a trial’s eligibility criteria. To address this challenge, the CCTS Hub joined TriNetX, a clinical data network of healthcare providers, pharmaceutical companies, and contract research organizations that connects clinical researchers to trial opportunities based on the patient populations they wish to study.
TriNetX uses the i2b2 (Informatics for Integrating Biology and the Bedside) framework, which is designed to enable enterprise-wide searches of de-identified health information. Researchers can use i2b2 to determine aspects of study feasibility by identifying whether a sizable number of patients exist that meet their study inclusion/exclusion criteria (CCTS offers a hands-on i2b2 training that teaches this and more). The TriNetX network does the same thing but across several dozen linked institutional i2b2 instances, including those at CCTS Partners UAB and Tulane, to provide query capability into the data of potentially millions of patients.
TriNetX increases clinical and translational research opportunities in two ways:
-
When a collaborating sponsor would like to stand up a clinical trial, it approaches TriNetX and requests a query of the electronic health records of all participating academic medical institutions via i2b2. Those sites with sufficient patients that meet eligibility criteria are contacted to invite participation. When UAB is identified as having a sufficient patient population for a given trial, the CCTS Research Commons fields the request, reaching out to the investigator-base at the Hub to identify potential site leads.
-
TriNetX also provides a foundation to support collaboration with peers and other member institutions. Using the same data framework and connections, academic sites can exchange data with each other to promote multisite investigation. The CCTS has created such a network with the University of Kentucky and is in the process of establishing similar capacities with other CTSA Hubs, including Emory, Medical University of South Carolina, and University of Arkansas. Additional sites are expected to come online throughout 2017, including CCTS Partners.
Advantages
Through TriNetX, CCTS investigators benefit from increased opportunities to participate in industry-sponsored clinical trials that are feasible and of scientific interest. Since going live on the TriNetX network in December 2015, the CCTS Hub has experienced a steady increase of trial opportunities, from one a month to weekly inquiries. These studies cover a range of diseases, including cancer, neurologic conditions, diabetes, inflammatory conditions, and genetic diseases. As a result of this partnership, trials have begun in Multiple Sclerosis and Nasopharyngeal Cancer under the leadership of Dr. Khurram Bashir and Dr. Lisle Nabell, respectively. Several other trials are pending.
Those trial units or investigators who are interested in learning more about trial opportunities are invited to contact Research Commons (
This email address is being protected from spambots. You need JavaScript enabled to view it. ). TriNetx Flyer
-
-
UAB-HudsonAlpha Center for Genomic Medicine
UAB-HudsonAlpha Center for Genomic Medicine
The Center for Genomic Medicine was established by two CCTS Partners, UAB and the HudsonAlpha Institute for Biotechnology, in support of genomics research to accelerate the understanding of the onset and progression of disease, unravel the mysteries of complex disorders, and enable new approaches to their prevention, diagnosis, and treatment. The Center brings together specialists whose collaborations fuel genomic discoveries and "propel these discoveries into clinical practice to develop true personalized therapies and cures." Learn more.
-
Undiagnosed Diseases Program
Undiagnosed Diseases Program
Through our signature Undiagnosed Patient Program (UDP), the CCTS works with partners across the network to evaluate patients with chronic, undiagnosed diseases. By providing access to sophisticated DNA sequencing (whole genome and/or whole exome) and a multidisciplinary medical team, we help find answers for patients with rare or unusual conditions that have defied diagnosis. To date, 81 of the 201 patients evaluated by our UDP have been successfully diagnosed.

To learn more about our other Genomic Medicine initiatives, click here.
