Accelerating Discovery to Improve Human Health
 Expert Trial Support from Start Up to Close Out
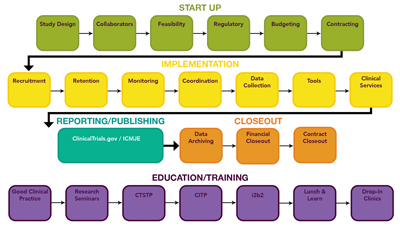
Expert Trial Support from Start Up to Close Out
The CCTS supports cutting-edge expertise and facilities for investigators conducting human subjects research. Our cost-effective, quality services exemplify best practice for every stage of the clinical research study lifecycle, from start up through implementation to close out.
Our Clinical Translation staff also offers trainings, from a lunch and learn series to a 6-month certificate program in the latest clinical and translational research competencies, to strengthen the research skills of every member of your team.
As a partner network, CCTS also connects you to a broad array of unique research resources across the Alabama-Louisiana-Mississippi region, such as the 7T MRI at Auburn University, the Genome Sequencing Lab at HudsonAlpha Institute of Biotechnology, and small molecule high-throughput drug lead screening at Southern Research.
Do you need immediate assistance with your clinical study? Contact us at
