Clinical History:
17-year-old primigravida with delivery of live born infant at 31 weeks gestation. Gestation complicated by non-immune hydrops (NIH) of unknown etiology, and severe preeclampsia. Placenta 526 g (269 - 411 g exp). What is the most likely cause of the NIH?
- Rh-isoimmunization
- Herpes Simplex Virus
- Listeria monocytogenes
- Cytomegalovirus
- Toxoplasma gondii
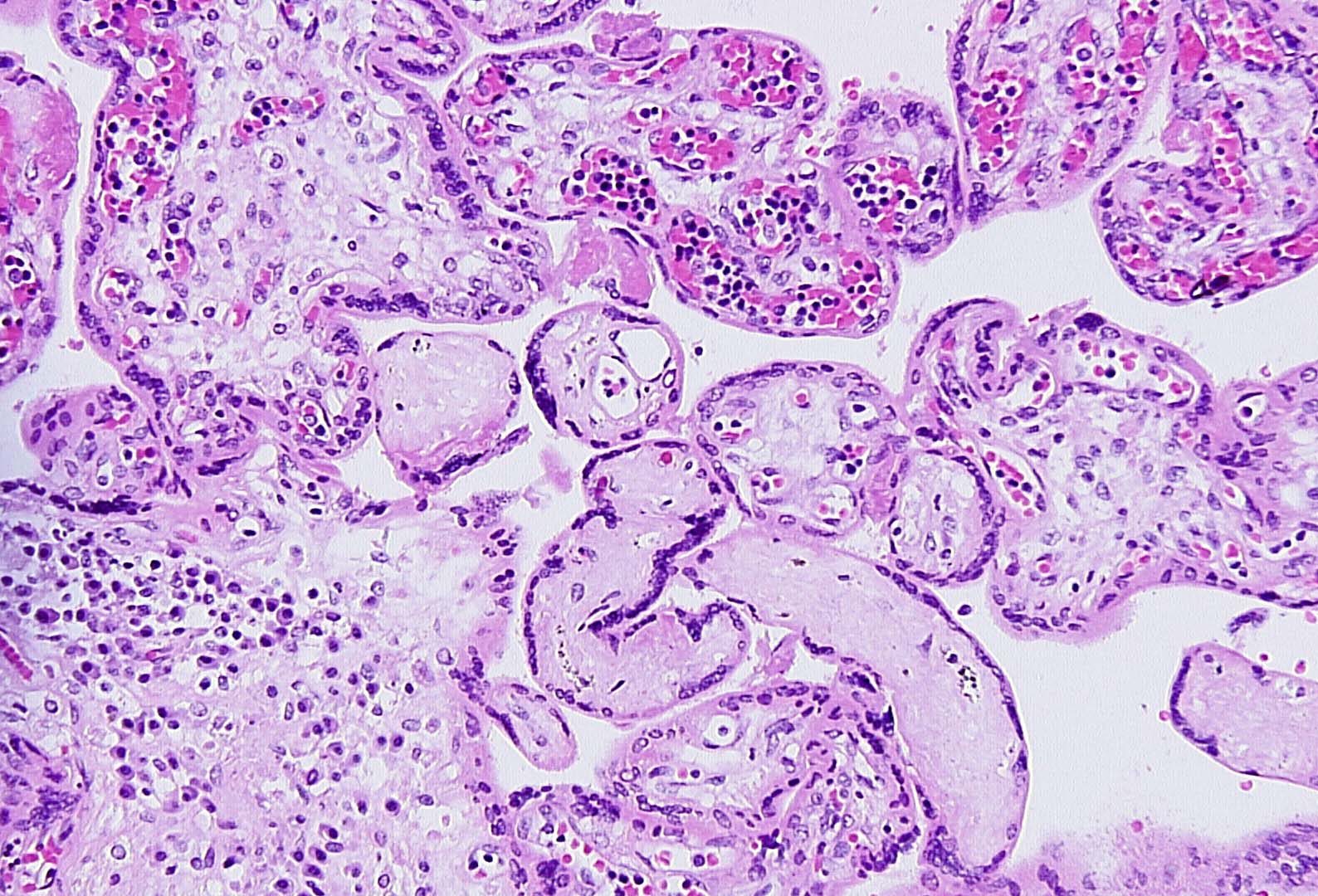
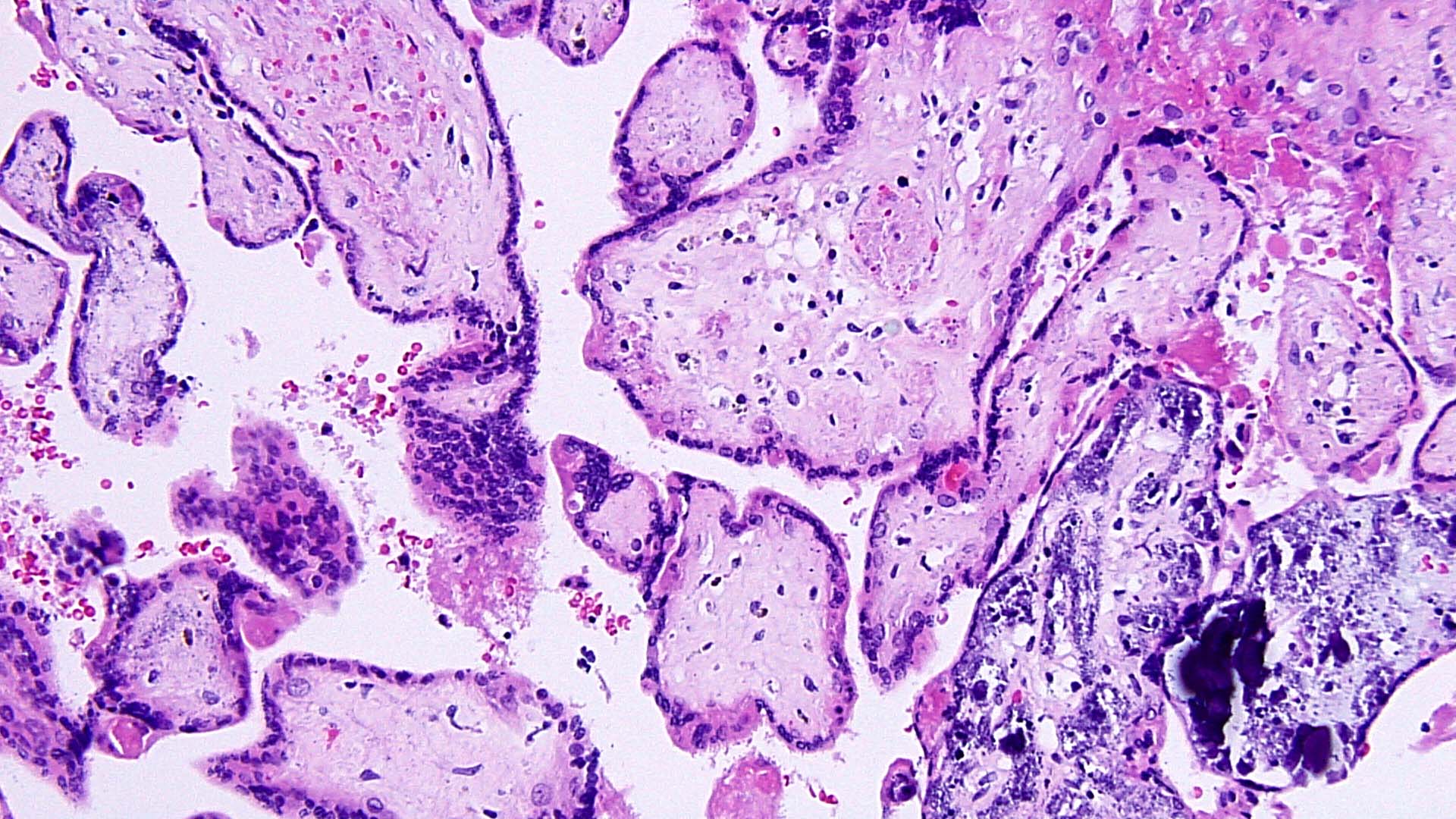
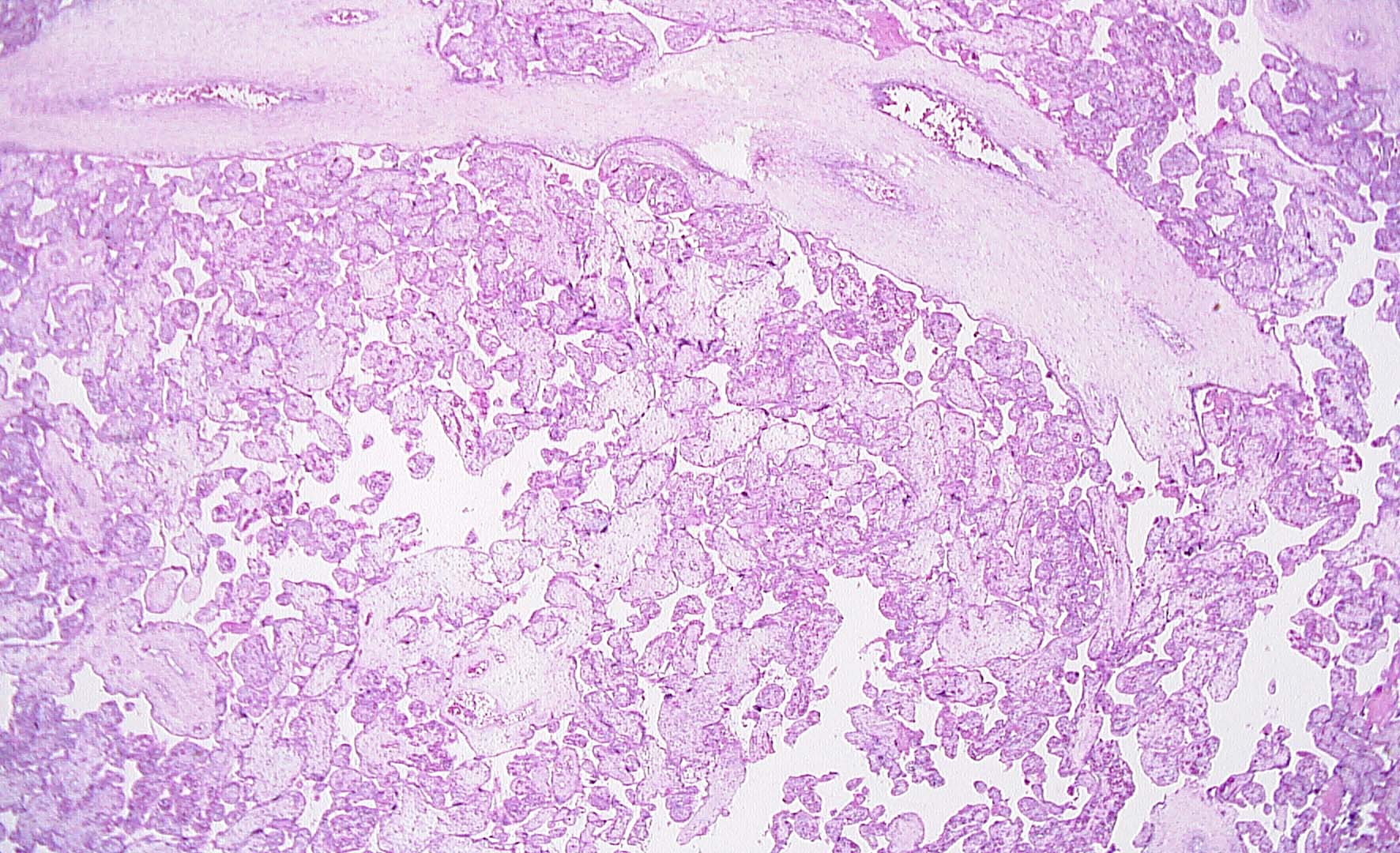
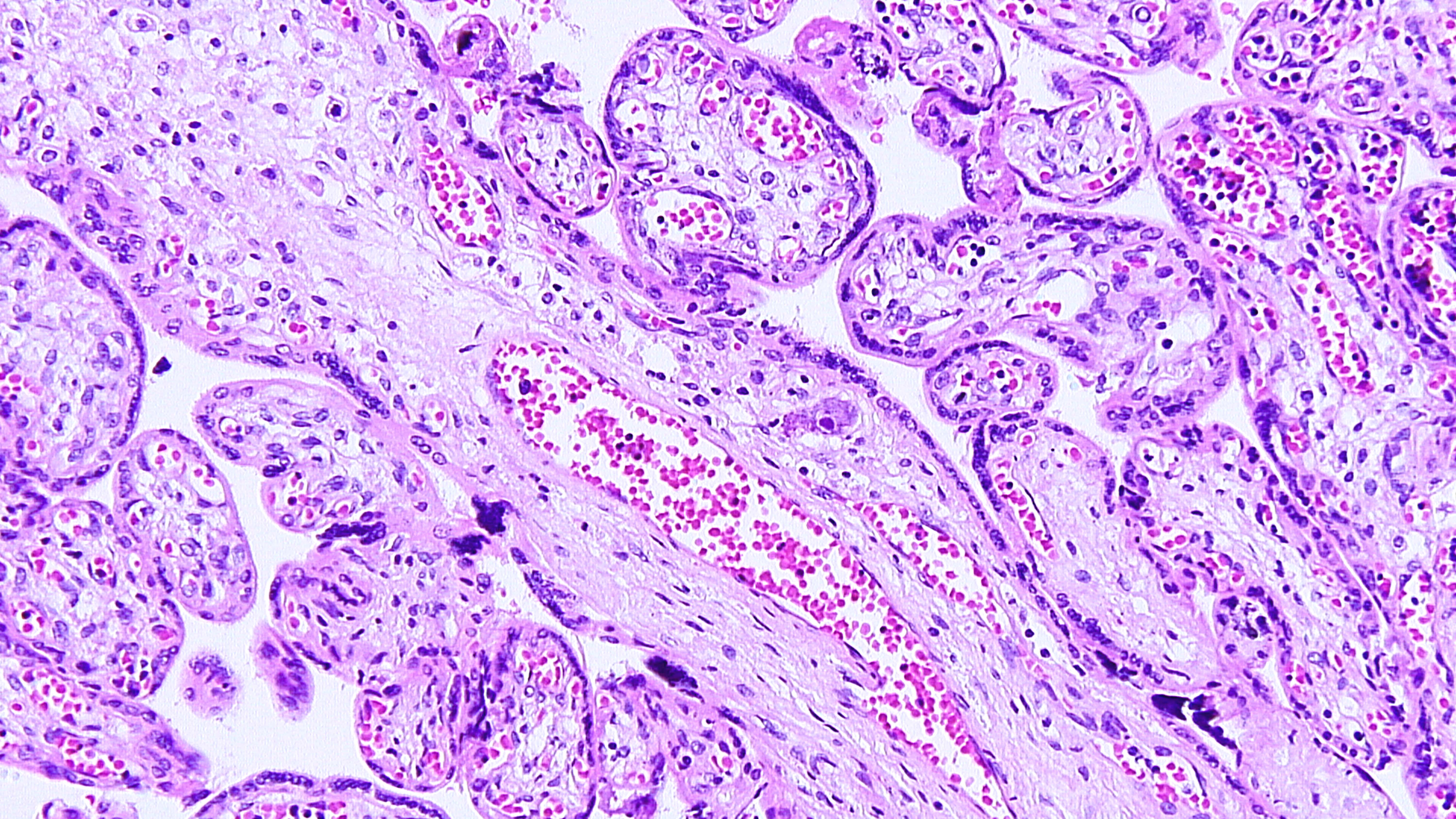
Maternal primary Cytomegalovirus infection with can result in hematogenous (vertical) transmission to the fetus and disseminated infection involving the liver, spleen, lungs, kidneys, bone marrow, brain, and endocrine organs. Acute, even disseminated infection in the fetus may not be accompanied by easily identified CMV “owl-eye” inclusions in the chorionic villi. CMV infects macrophages/monocytes/ CV Hofbauer cells, epithelial cells, and endothelial cells. The slides show features highly concerning for intrauterine CMV infection and should prompt very careful search for CMV inclusions. Findings of lymphoplasmacytic stromal infiltrate (which can also be difficult to find), hemosiderin deposition (Fig 1), villous sclerosis, and scattered calcifications (“tombstones”) (Figs 1, 2) are major clues. Patchy chorionic villous edema (Fig 3) (histology of hydropic placentomegaly in clinical history), Hofbauer cell hyperplasia, and fetal erythroblastosis (many circulating immature RBCs) (Fig 1) are also common. CMV intranuclear inclusions can be very sparsely distributed. (Fig 4) Fig 5 is a higher power image of the inclusion in Fig 4 and Fig 6 shows the confirmatory positive CMV immunostain of a cluster of infected cells. CMV immunostain also aided in revealing sparely scattered infected cells in this case. Infected endothelial cells die when virus released and vessel integrity is compromised allowing for thrombosis and extravasation of fetal RBCs out into the vessel wall and stroma (CMV is one cause of fetal vascular malperfusion lesions) (Figs 2, 3) and further distal villous sclerosis and hemosiderosis and calcification in dependent villi. Fig 7 shows thrombosis in larger stem villi, calcifications and villous sclerosis. Fig 8 shows an unusual finding of infected trophoblast cells along with pale, refractile hemosiderin deposition.
A: Rh-isoimmunization would result in erythroblastosis fetalis but not in villous inflammation. B. HSV infection when hematogenously spread is characterized by lymphoplasmacytic villitis but there is associated villous trophoblast necrosis and fibrin and cellular debris in the perivillous space(“dirty background”); HSV immunostain is positive. C: Listeria monocytogenes shows aggregates of acute inflammation in the perivillous space (acute intervillositis) and acute villitis; Gram positive bacilli can be seen in the villi and in the inflamed membranes. E: T. gondii is characterized by a very destructive lymphoplasmacytic villitis and intervillositis due to invasive form of the organism (tachyzoites.) Pseudocysts (macrophages containing the bradyzoites) are also detectable in the villi.
However, pseudocysts are best seen in the umbilical cord, due to the low cellularity of Wharton’s jelly; Toxoplasma immunostain will reveal the presence of both tachyzoites and bradyzoites.
Case contributed by Ona Marie Faye-Petersen, M.D., Professor, Anatomic Pathology