Case History
Placenta, 24-year-old P3013 at 28 5/7th weeks. Placental weight 641 g (210 – 331 g expected). Fetal hydrops and intrauterine demise.
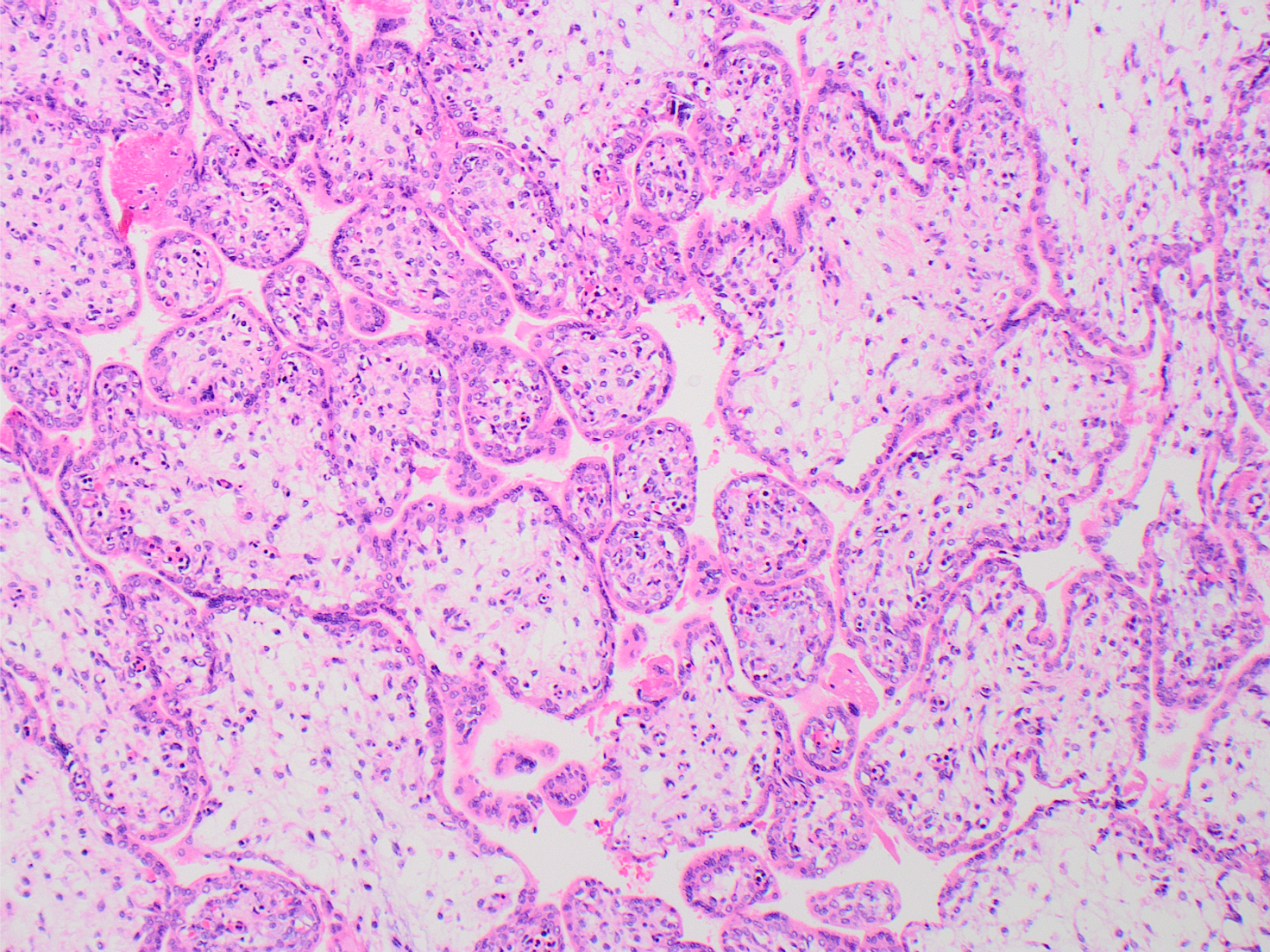
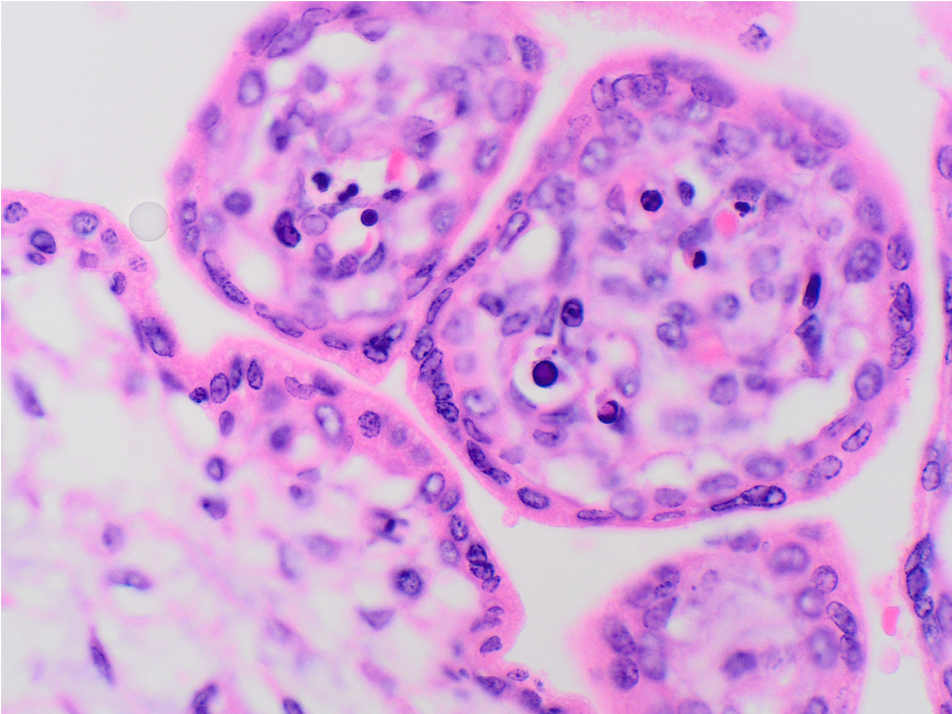
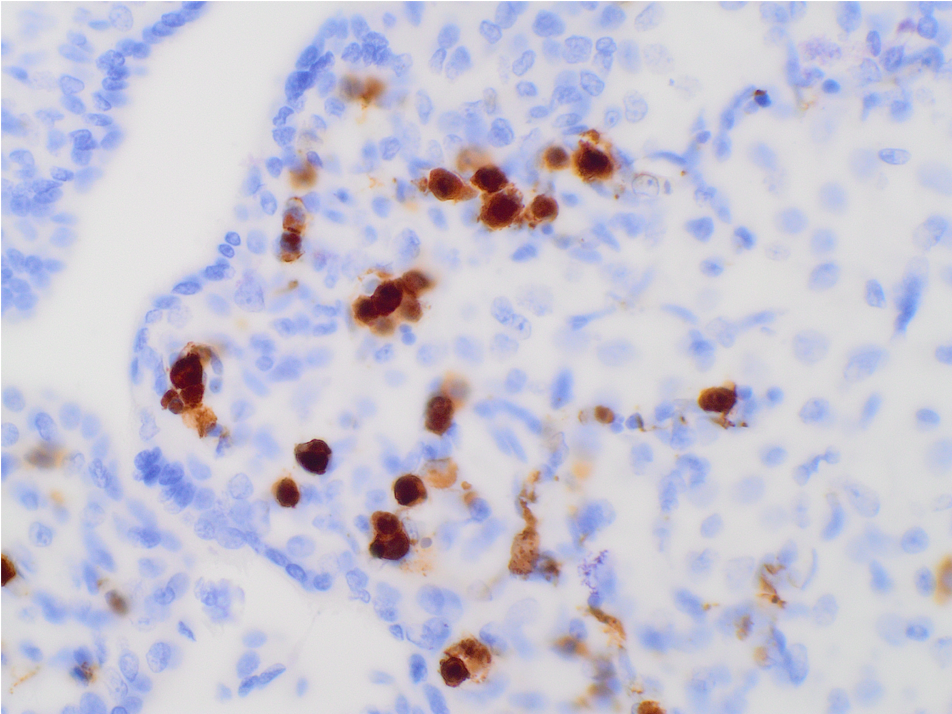
What is the diagnosis?
- Cytomegalovirus infection
- Herpes simplex virus infection
- Listeria monocytogenes infection
- Fetal transient abnormal myelopoiesis (TAM)
- Parvovirus B-19 infection
Answer: E - Parvovirus B-19 infection
The low power H&E view shows early 3rd trimester chorionic villi with stromal edema and many intracapillary hematopoietic precursors, visible even at low power. High power H&E view shows intravillous capillaries containing early erythroid precursors with nuclear viral cytopathy characterized by smudged and marginated chromatin. Immunostain for parvovirus confirms viral inclusions within cells in intracapillary spaces.
Ultrasound at 18 weeks was within normal limits. However, at 28 weeks the fetus was noted to be hydropic with massive skin edema, pleural effusions, pericardial effusions and placentomegaly. Intrauterine demise occurred shortly thereafter. Hydrops fetalis results from a disturbance in fluid homeostasis and has a wide array of potential etiologies. “Non-immune” hydrops fetalis (NIH) refers to hydrops caused by any etiology other than maternal anti-fetal red blood cell hemolysis (“isoimmune” hydrops fetalis). Historically, isoimmune hydrops fetalis was more common, but it has decreased sharply since the widespread availability of anti-D gamma globulin prophylaxis.
PB19 is one of the more common causes of NIH. PB19 infects erythroid precursors, directly suppressing erythropoiesis, and causing fetal anemia. The severity of anemia determines the severity of hydrops. Anemia leads to increased hydrostatic pressure within capillary beds, forcing fluid into the interstitium. Progressive cardiac failure contributes to this due to decreased myocardial contractility. The “third spacing” is further exacerbated by decreased oncotic pressure as hypoxemia leads to increased protein leakage across damaged capillaries. These fluid shifts are also manifest in the placenta by villous edema and placentomegaly (which is often marked).
PB19) is a single-stranded DNA virus, spread by respiratory secretions. Among previously seronegative pregnant women, in a reported 17-22% of cases the virus may be vertically transmitted to the fetus. In most of these fetuses, the infection will spontaneously resolve, but a subset go on to develop clinically significant anemia and may require intrauterine transfusion or may result in miscarriage or stillbirth. In the classic scenario, the mother reports sick contacts or respiratory symptoms, but this is not always the reality.
In this case, the mother had no symptoms or known sick contacts and viral illness was not suspected; placental pathology provided the etiology of the IUFD. Placental pathology can be very helpful in such cases to inform pregnancy counseling and reassure the family that recurrence of NIH in future pregnancies is low.
Bascietto F, Liberati M, Murgano D, et al. Outcome of fetuses with congenital parvovirus B19 infection: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;52(5):569-576.
Randenberg AL. Nonimmune hydrops fetalis part I: etiology and pathophysiology. Neonatal Netw. 2010;29(5):281-295.
Case contributed by: Virginia Duncan, M.D.,Assistant Professor, Anatomic Pathology, Chief, Pathology and Laboratory Medicine, Birmingham VAMC