Case History
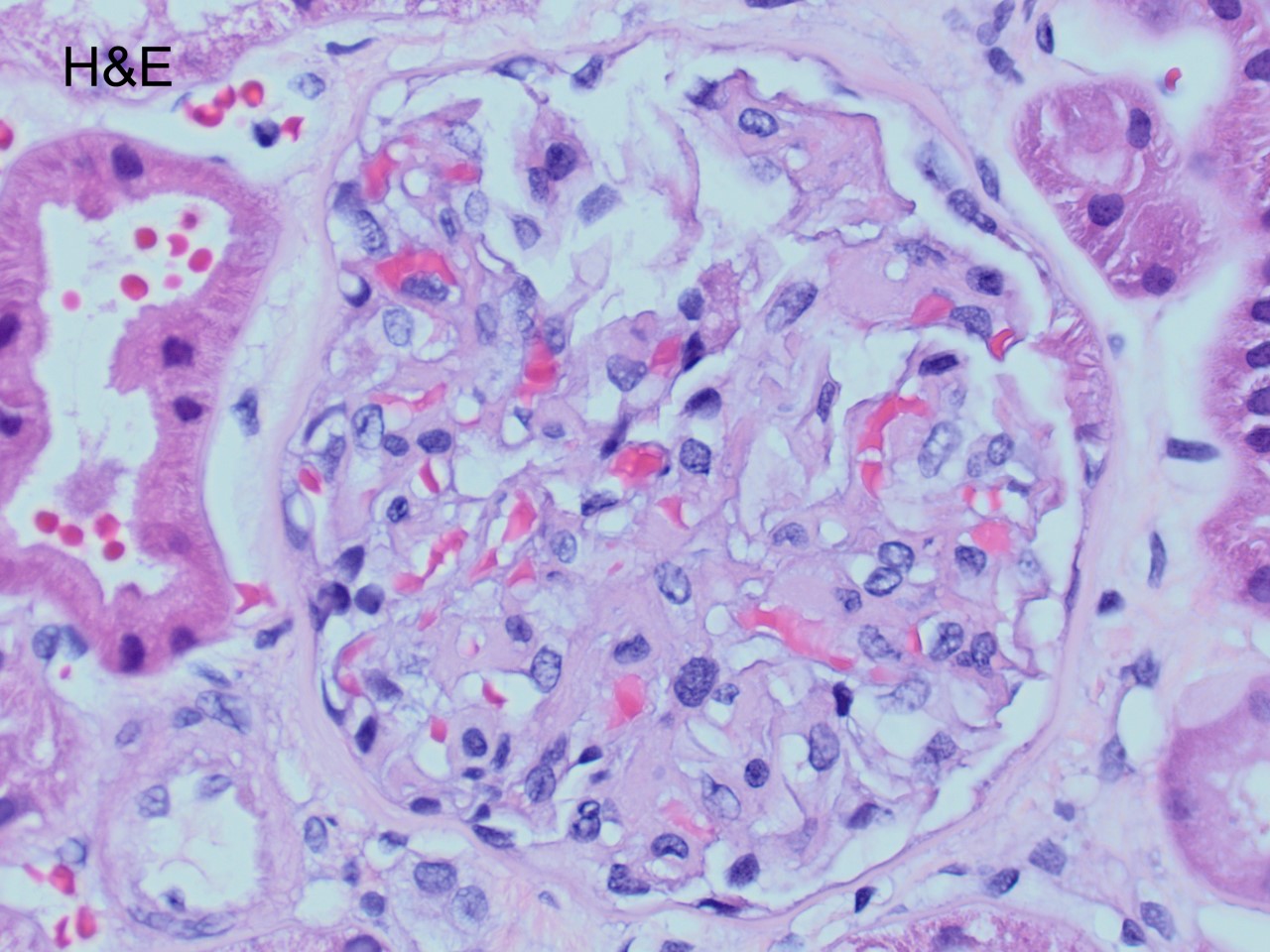
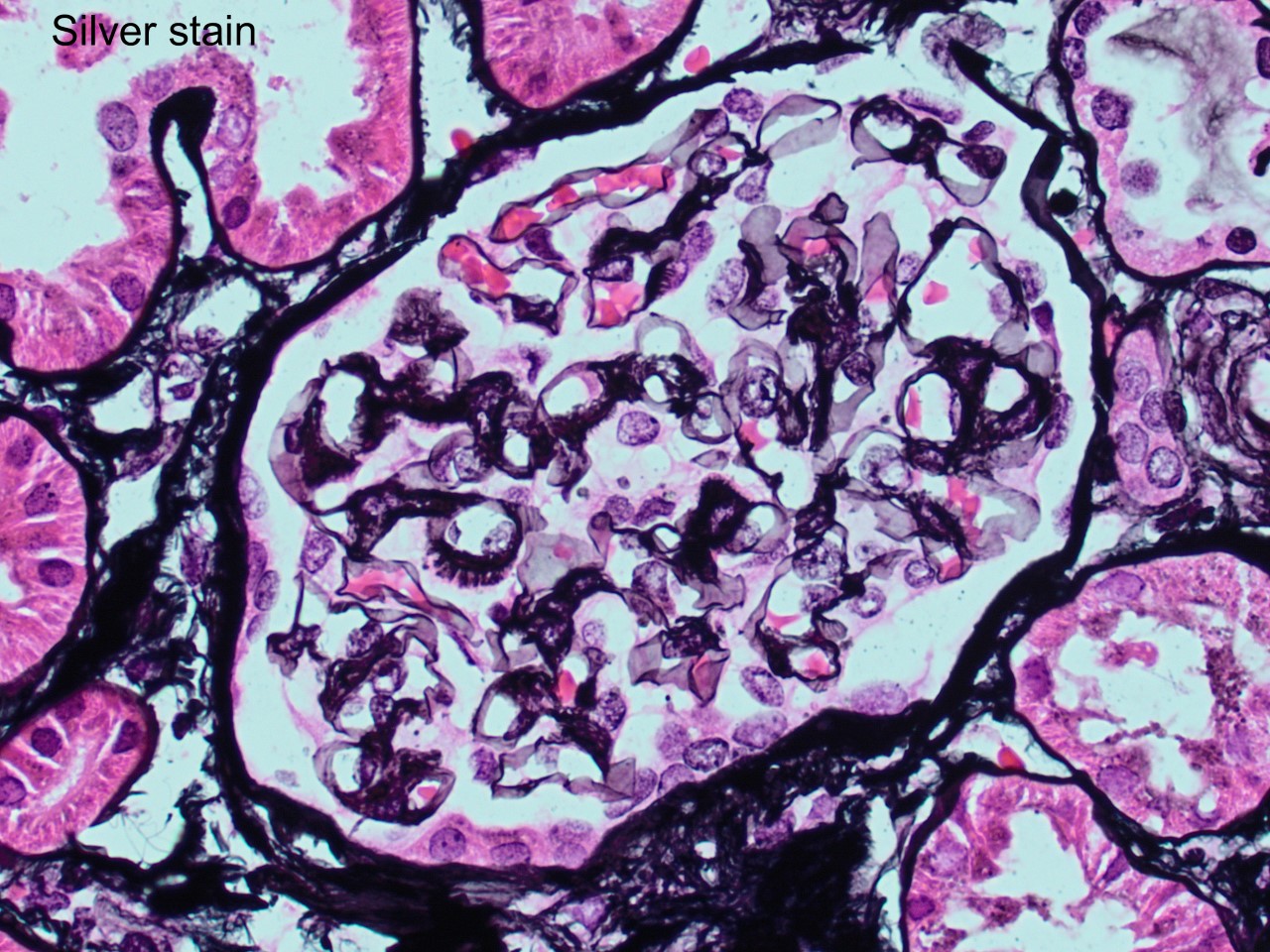
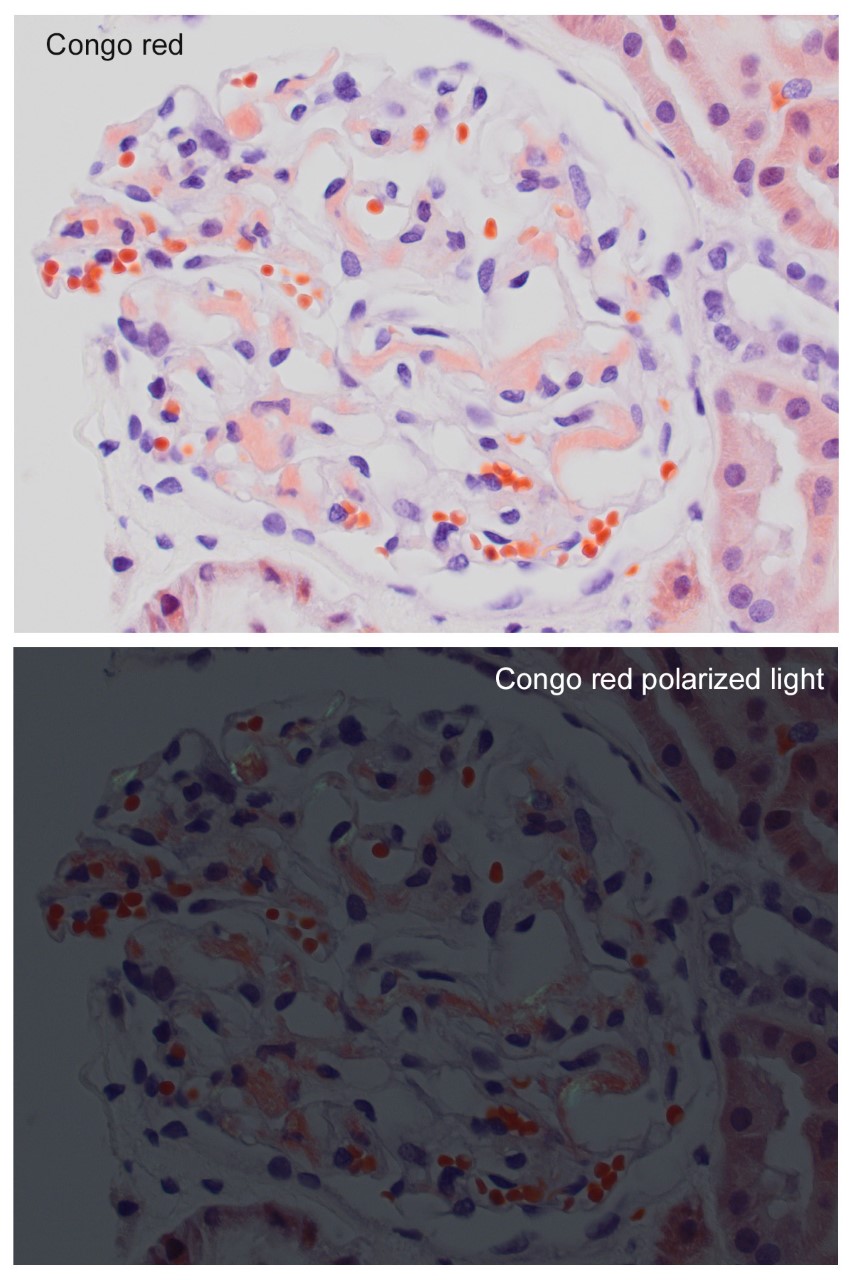
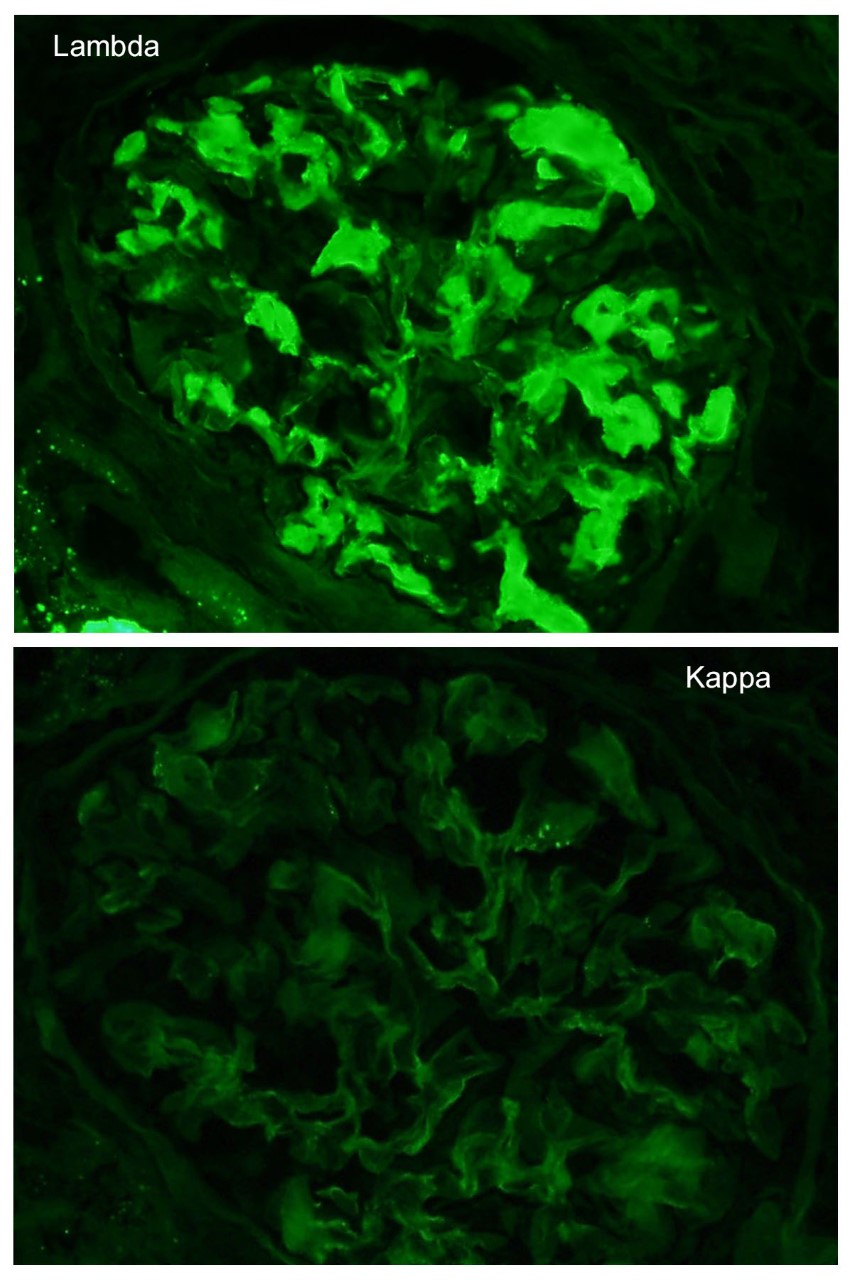
A 52 year-old asymptomatic woman was found to have nephrotic range proteinuria, mild hypoalbuminemia, and normal renal function. A renal biopsy was performed. H&E, silver stain, Congo red stain, lambda and kappa light chains immunofluorescence are shown. Remaining IF negative.
What are the expected ultrastructural findings?
- Parallel microtubules
- Nonbranching fibrils ~ 10 nm
- Curved annular structures
- Finely granular deposits
Correct Answer: B. Nonbranching fibrils ~ 10 nm
The patient has AL amyloidosis involving the glomerular compartment. The light microscopy and immunofluorescence features in this case are characteristic with mesangial expansion by acellular pale eosinophilic amorphous material, segmentally along the capillary wall, pale with PASD, occasional feathery spikes with silver stain, apple green birefringence under polarized light with Congo red, and monotypic smudgy staining with lambda light chain by immunofluorescence.
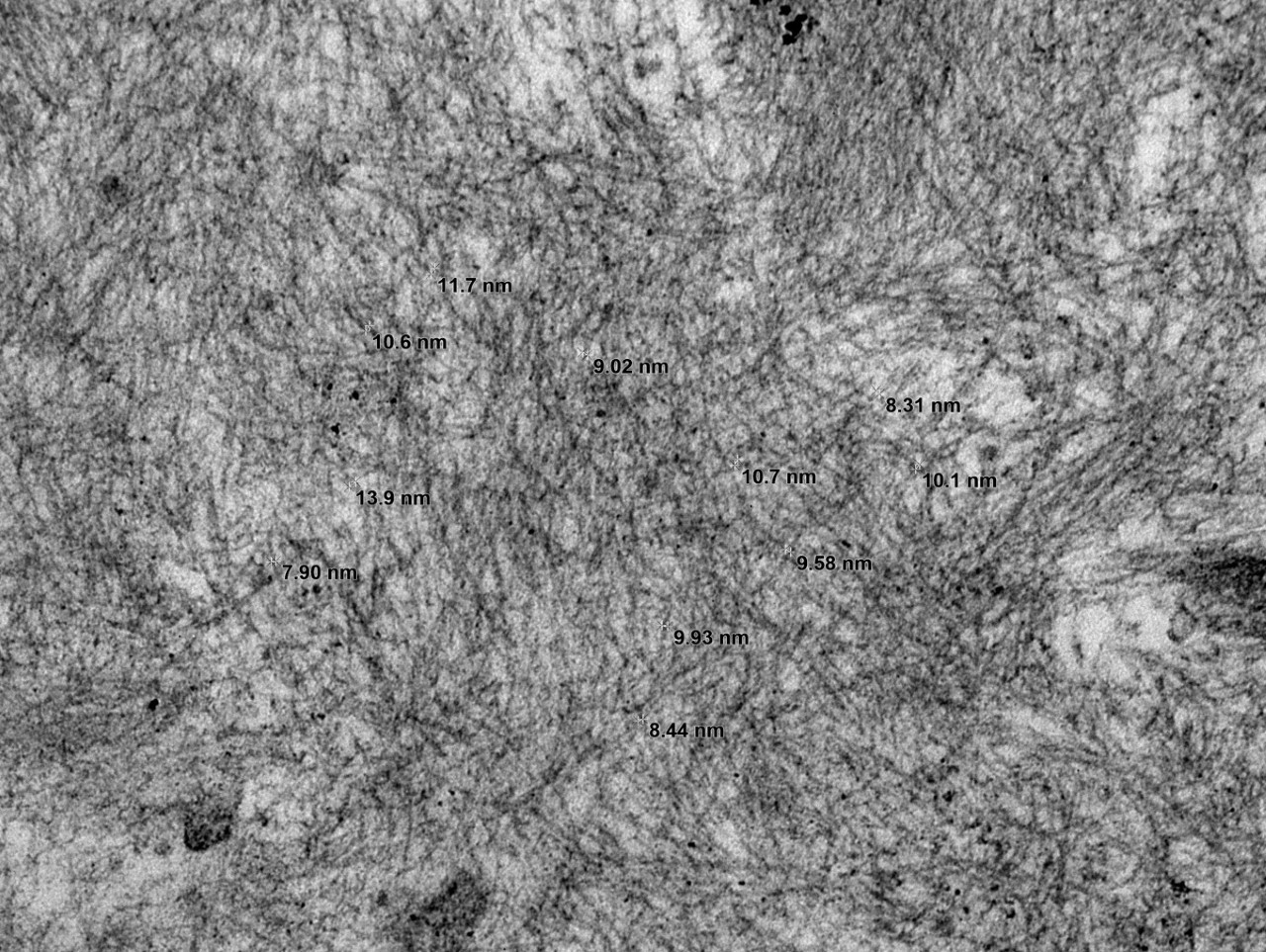
The ultrastructural findings confirmed the diagnosis with the presence of randomly arranged non-branching fibrils measuring 8-14 nm in diameter (Figure), in the mesangium and along the basement membrane. Mass spectrometry confirmed AL-type amyloid. A subsequent bone marrow evaluation showed 7% atypical monoclonal plasma cells (plasma cell dyscrasia).
Amyloidosis is characterized by the extracellular deposition of amyloidogenic proteins with a beta-pleated sheet tertiary structure that are resistant to degradation. The type of amyloid can be determined by immunohistology and/or mass spectrometry. The majority of the cases of renal amyloidosis are due to involvement by AL or AA forms. The patients are typically between 50-70 years of age and present with proteinuria, usually in the nephrotic range. The prognosis and treatment depend on the type of amyloid and underlying associated condition.
The distinction between renal amyloidosis and other diseases with monoclonal immunoglobulin deposits is based on morphology, Congo red stain, and electron microscopy findings. When Congo red is positive, the diagnosis of amyloidosis is confirmed. If negative, ultrastructural findings aid in the diagnosis: amyloid - randomly arranged, non-branching fibrils 8-12 nm in width; immunotactoid glomerulopathy - parallel arrays of hollow microtubules typically >30 nm in diameter; type I cryoglobulinemia - curvilinear, short, annular or microtubular structures typically >30 nm; proliferative GN with monoclonal immunoglobulin deposits (PGNMID) – amorphous electron dense deposits; monoclonal immunoglobulin deposition disease (MIDD)- finely granular electron dense deposits.
References:
Secondary glomerular diseases (2017). In: Fogo AB, Kashgarian M. (eds). Diagnostic atlas of renal pathology. 3rd ed. Elsevier. Philadelphia, PA. pp 147-160.
Renal disease (2013). In: Stirling JW, Curry A, Eyden B (eds). Diagnostic electron microscopy- A practical guide to interpretation and technique. 1st ed. Wiley. West Sussex, UK. pp 1-54.
Case contributed by: Andrea Kahn, M.D., Professor, Anatomic Pathology