Case History
Mass in the face of an adult man. Of the following, which genetic abnormality would you expect based on these images?
A. MDM2 amplification
B. BRAF V600
C. t(17;22) and/or COL1A1àPDGFB fusion protein
D. UV-signature mutations
Answer: C. t(17;22) and/or COL1A1àPDGFB fusion protein
Discussion:
Dermatofibrosarcoma protuberans (DFSP) is a nodular cutaneous neoplasm of spindle cells, characterized by storiform architecture and potential locally aggressive behavior. DFSP most commonly occur in the trunk (48%), followed by the extremities (38%), and the head and neck region (15%).
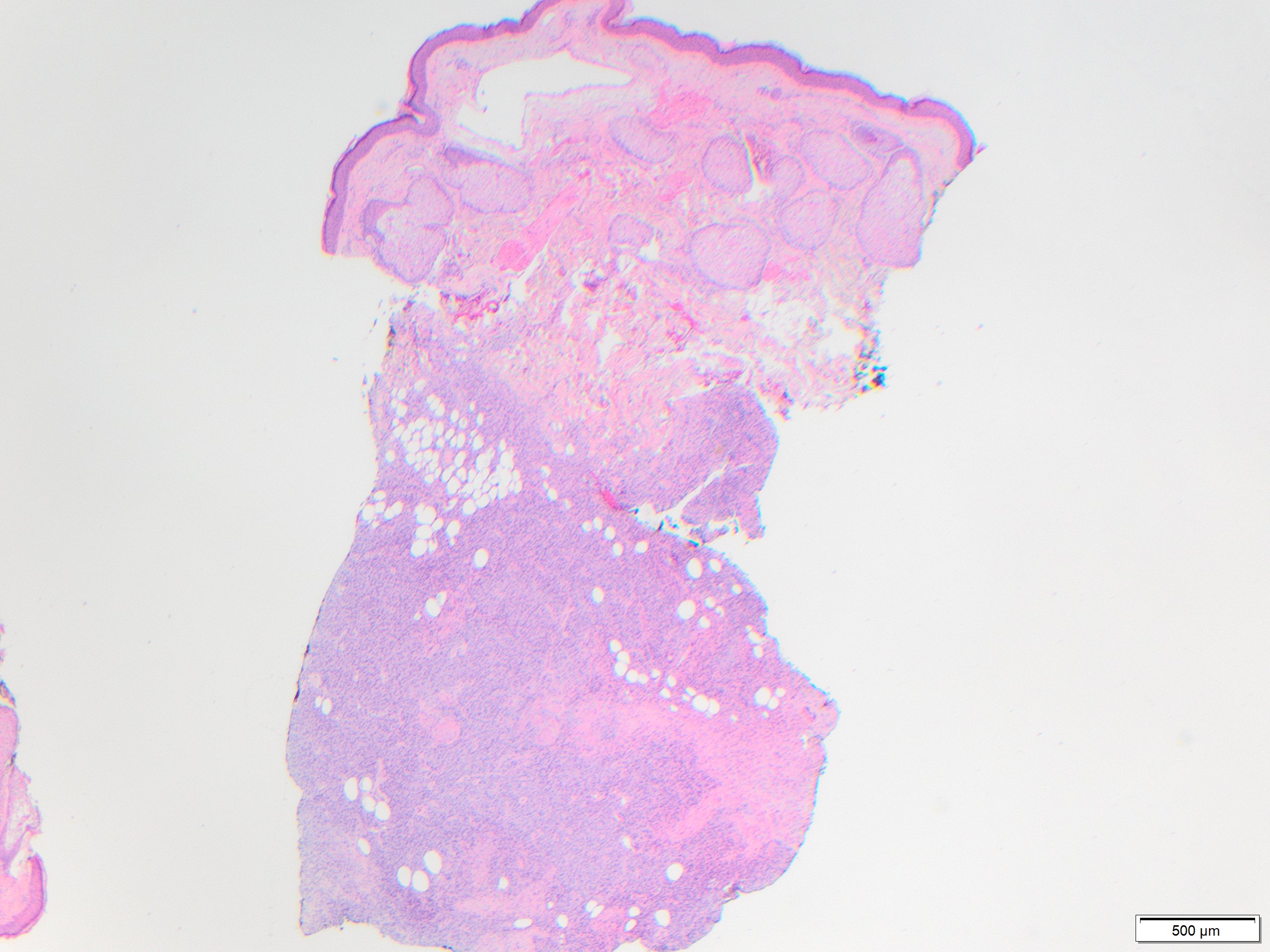
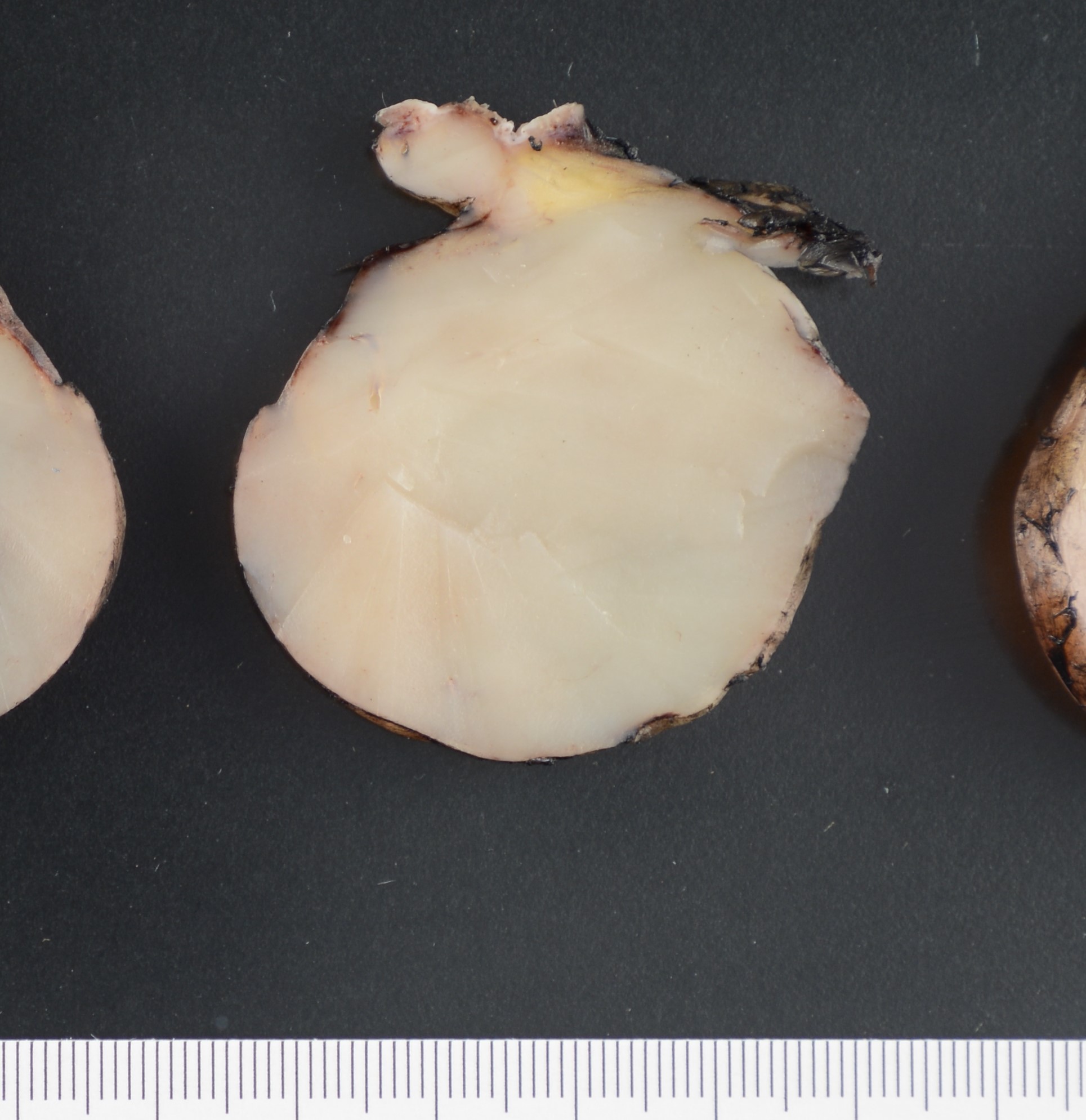
In early stage DFSP forms as a firm plaque-like lesion that grows slowly and later becomes nodular, hence “protuberant”. Most DFSP are biopsied during the nodular stage, consisting of a solitary-protuberant gray-mass involving subcutis and skin. Some tumors may be centered in the subcutaneous soft tissue with subtle dermal involvement. Therefore, involvement of the dermis may not always be evident in the initial biopsy, as seen in the present case.
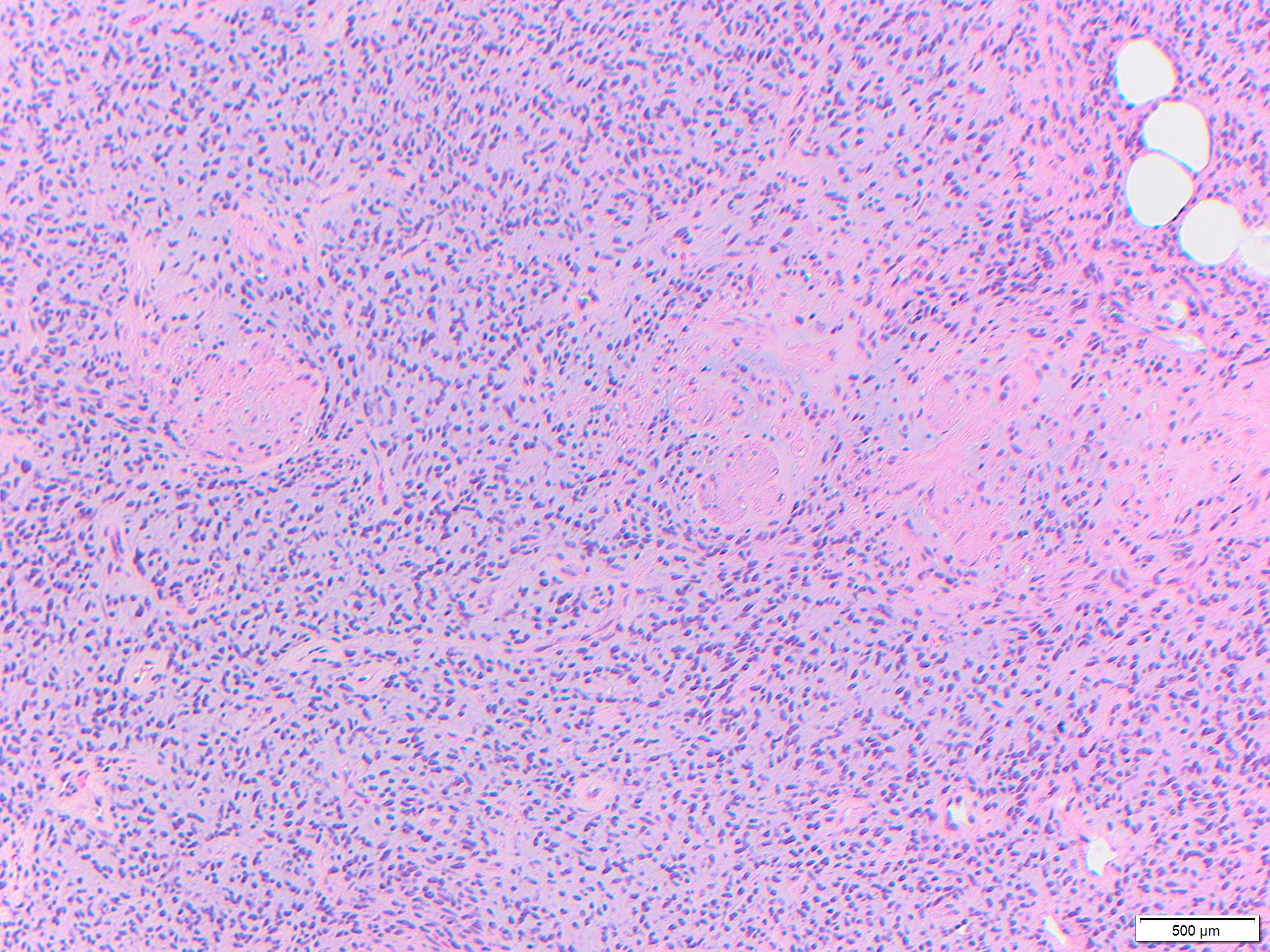
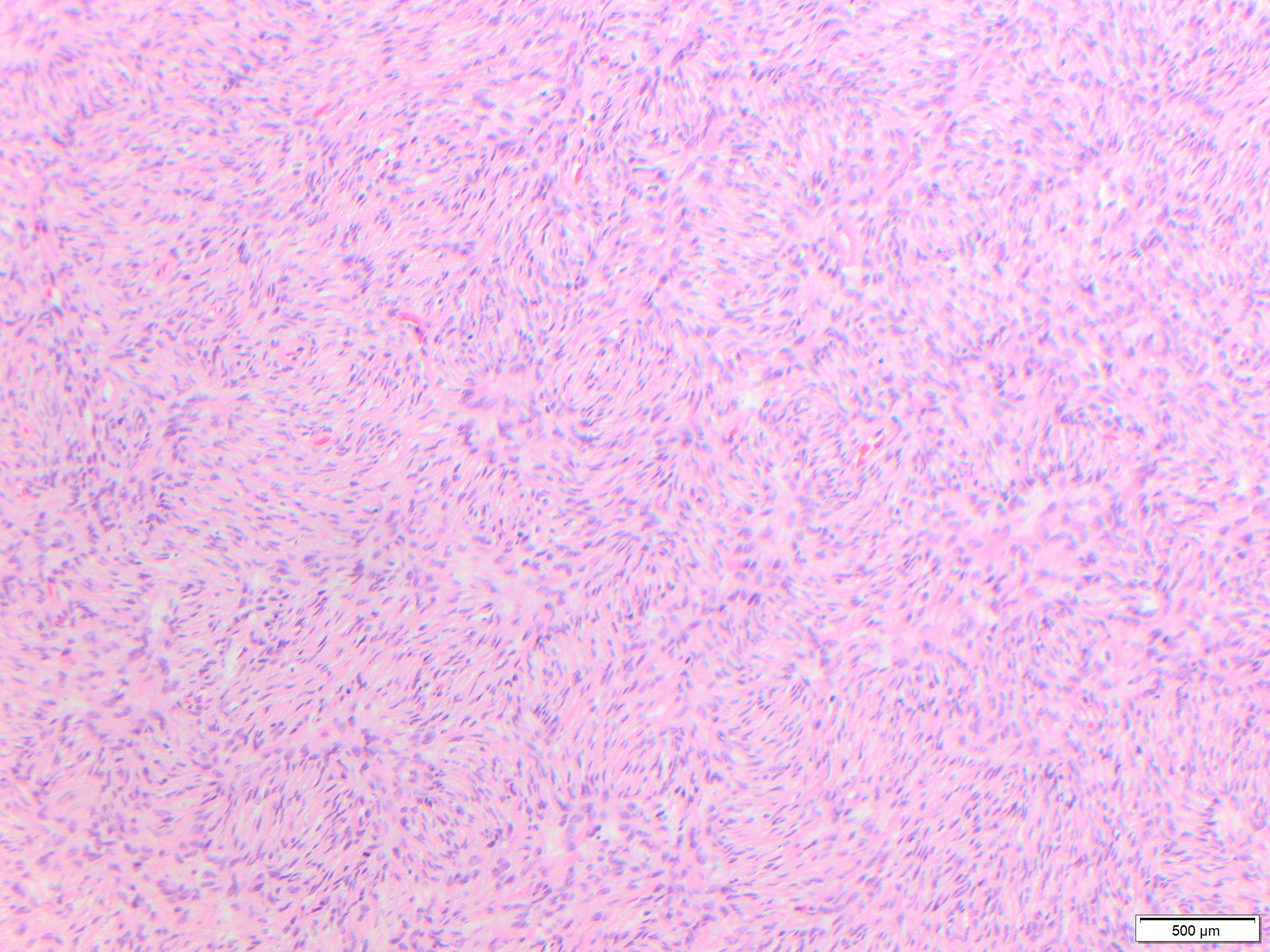
While these tumors may appear grossly circumscribed, microscopically DFSP are ill-defined with diffuse infiltration of dermis and subcutis. The tumor is composed of uniform spindled cells with collagenous stroma, often arranged in a storiform or “cartwheel” pattern, with infiltration of fat lobules and adnexal structures at the periphery. Some DFSP lack the collagenous storiform arrangement and instead display variably myxoid stroma, and some DFSP, like this example, may display myoid differentiation and so called “myoid-bodies” which may also be seen in other types of mesenchymal tumors. By immunohistochemistry DFSP are diffusely positive for CD34, and negative for S100, smooth muscle actin (SMA) and desmin, except myoid areas when present, are positive for SMA. Areas with herringbone architecture and loss of CD34 staining, may indicate fibrosarcomatous transformation. The histologic differential diagnosis of DFSP includes benign fibrous histiocytoma, neurofibroma, plaque-like CD34-positive dermal fibroma, and superficial acral fibromyxoma.
Most DFSP harbor the recurrent unbalanced chromosome translocation t(17;22)(q21;q13), commonly in the form of a supernumerary ring chromosome, resulting in a COL1A1-PDGFB fusion protein which can be detected by fluorescence in situ hybridization, reverse transcriptase-polymerase chain reaction, or next generation sequencing . A recent study by Cloutier et al showed the utility of performing chromogenic in situ hybridization in formalin-fixed paraffin embedded tissue as a faster diagnostic tool for DFSP.
Of the other answers, MDM2 amplification is seen in atypical lipomatous tumor, liposarcoma, osteosarcoma, intimal sarcoma, and some carcinomas. BRAFV600 mutations are seen in about half of melanomas. Finally, UV-signature mutations are mutations that meet a number of established criteria, and support a sun damage-induced mechanism of pathogenesis, thus more likely to be seen in squamous cell carcinoma, melanoma, and Merkel cell carcinoma. Sun damage as a factor for pathogenesis of DFSP has not been studied.
References:
Goldblum JR, Folpe AL, Weiss SW. MD Enzinger and Weiss’s Soft Tissue Tumors, 7th Edition: 2020. p 424
Cloutier JM, Allard G, Bean GR, Hornick JL, Charville GW. PDGFB RNA in situ hybridization for the diagnosis of dermatofibrosarcoma protuberans. Mod Pathol. 2021 Aug;34(8):1521-1529. doi: 10.1038/s41379-021-00800-2. Epub 2021 Mar 24. PMID: 33762682; PMCID: PMC8298273.
Calonje E, Fletcher CD. Myoid differentiation in dermatofibrosarcoma protuberans and its fibrosarcomatous variant: clinicopathologic analysis of 5 cases. J Cutan Pathol. 1996;23:30–36.