Case History
A 24 year old African American female with right upper quadrant abdominal pain & amenorrhea (2nd to Depo-Provera use for approximately 9 yrs.). CT scan showed poorly defined lesion at hepatic dome with irregular central hypoattenuation.
What is the diagnosis?
- Hepatic adenoma
- Scirrhous HCC
- C. Fibrolamellar hepatocellular carcinoma (FLC)
- Focal nodular hyperplasia (FNH)
- Cirrhotomimetic hepatocellular carcinoma
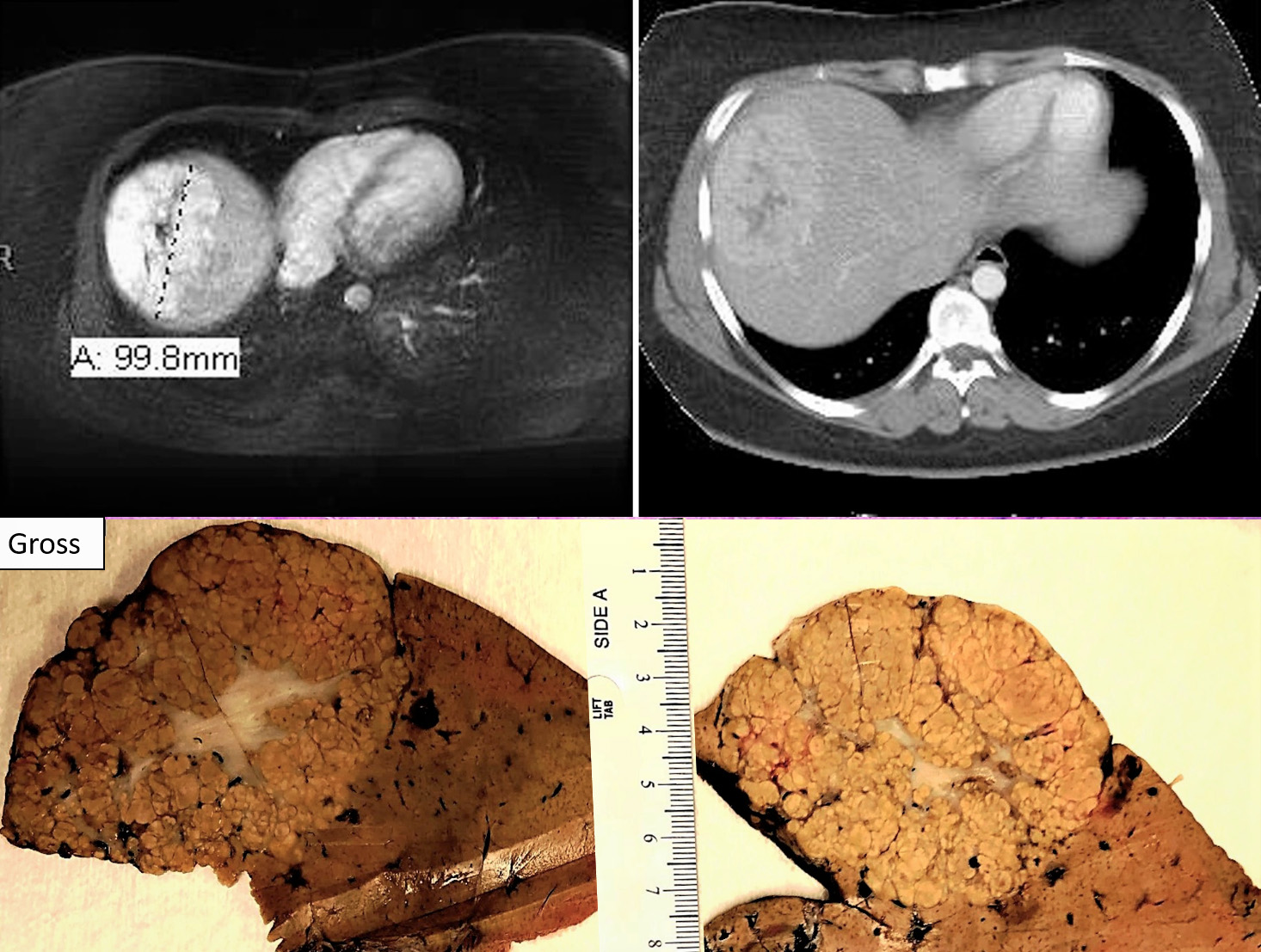
The answer is “D”: Focal nodular hyperplasia (FNH)
Discussion:
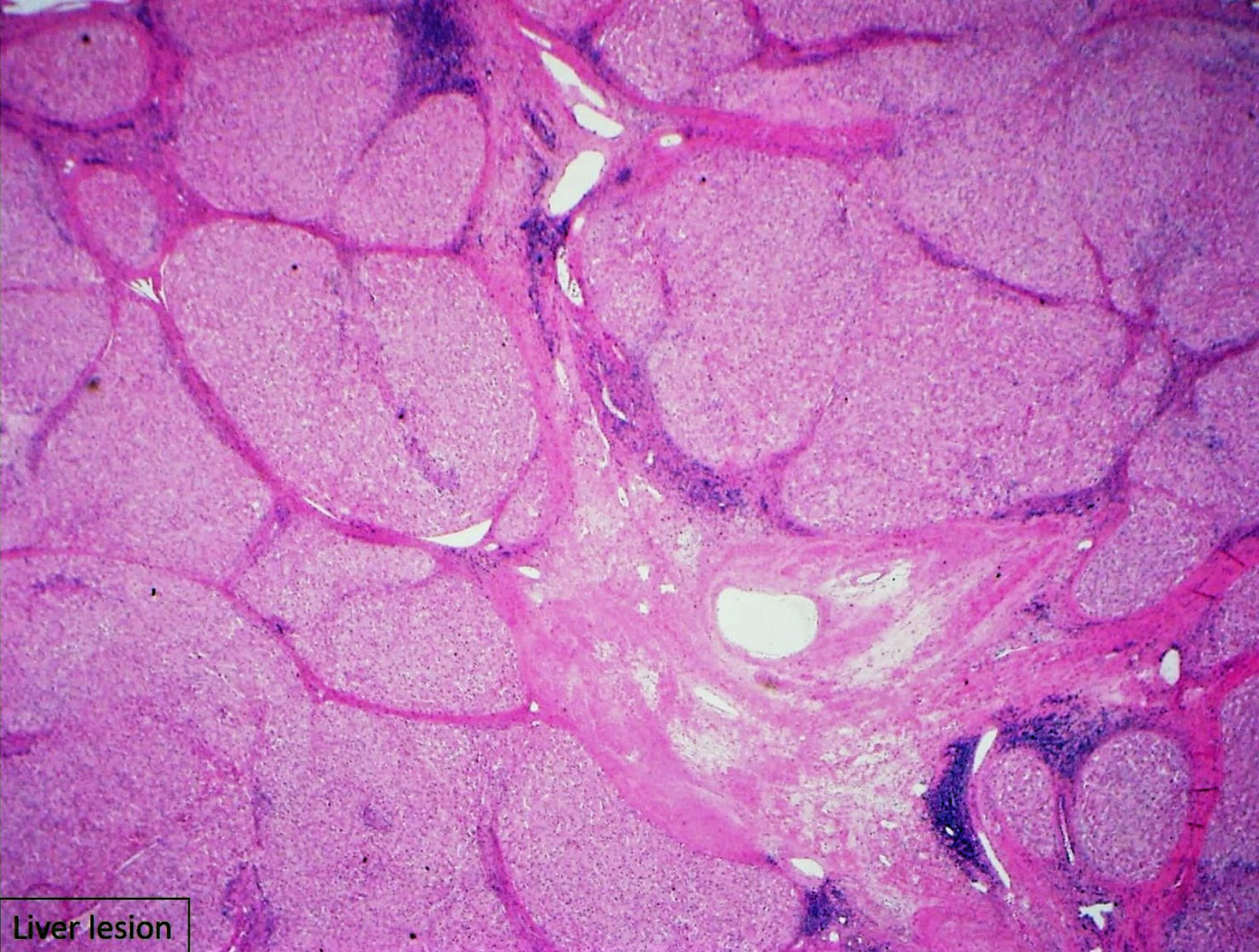
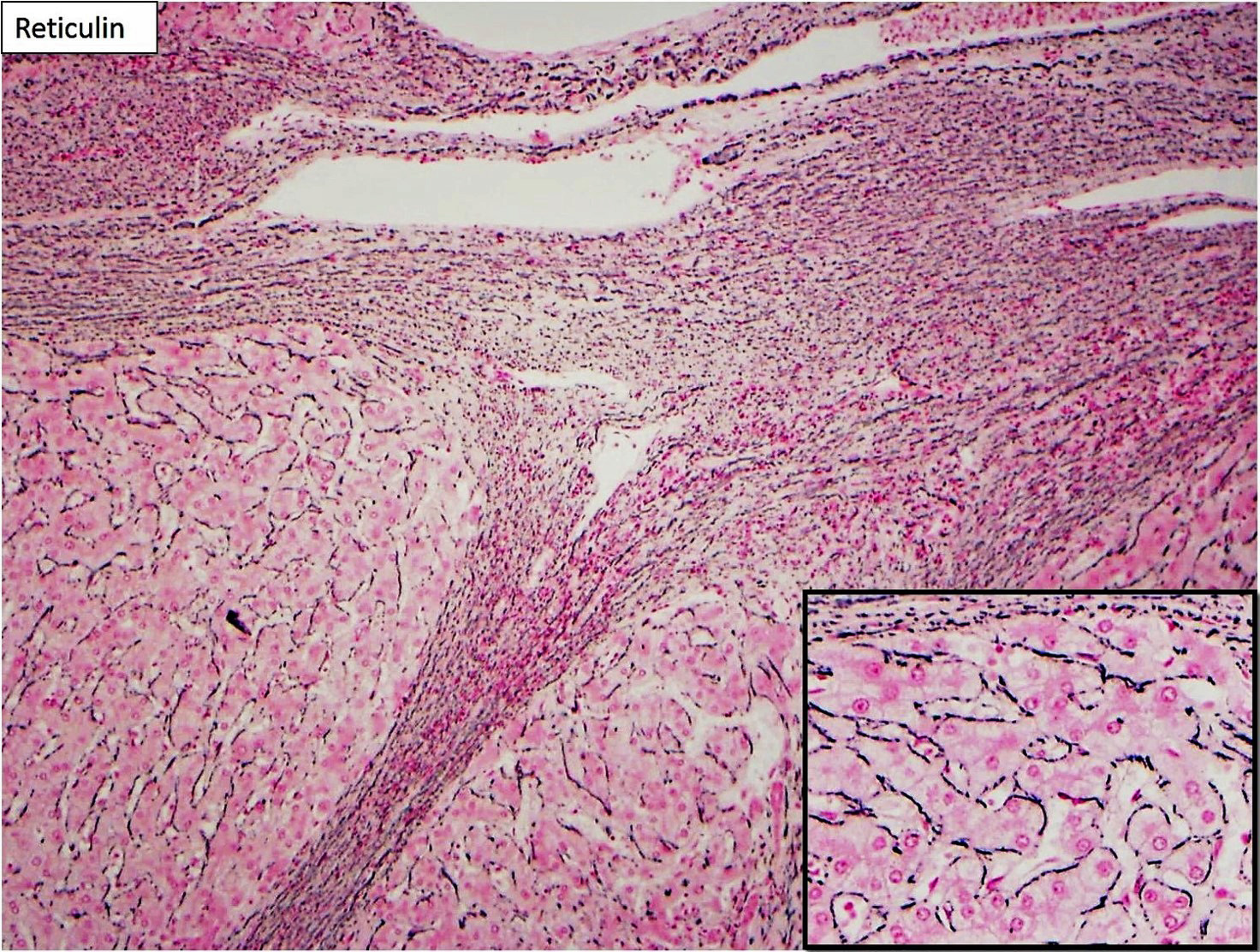
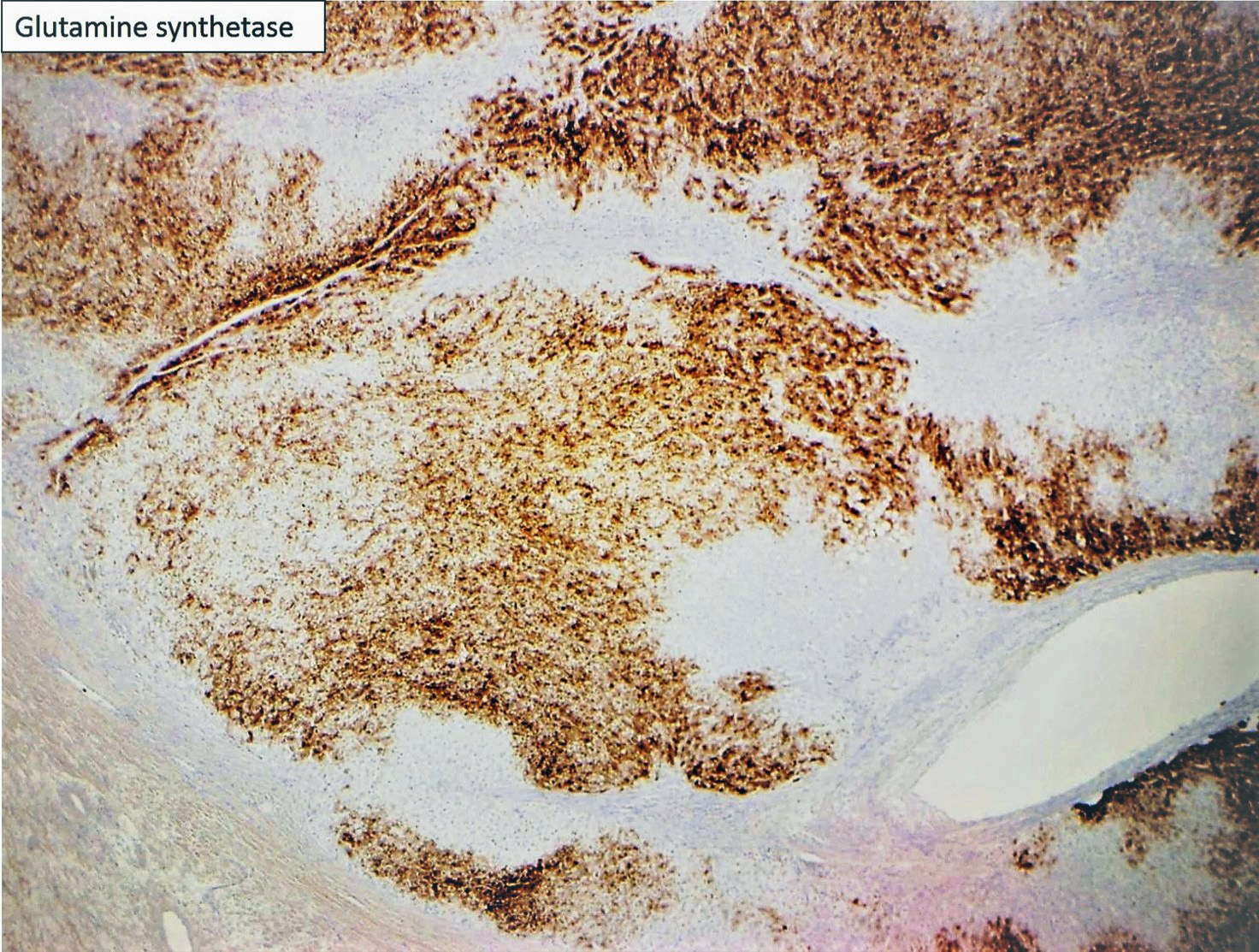
Focal nodular hyperplasia (FNH) is reactive lesion that is composed of hepatocytes and bands of fibrosis that occur due to vascular shunting in noncirrhotic liver. Usually FNH is a single lesion that most often seen in young and middle-aged women. Macroscopically, it is well-demarcated tan sub capsular mass with central stellate scar. Histologically, on low magnifications, it resembles cirrhosis with nodularity and band of fibrosis. The hepatocytes lack cytologic atypia. Multifocal areas of bile ductular proliferation (metaplasia) often seen at the interface of the hepatic parenchyma and the fibrous bands. Abnormal thick walled arteries are present in the center of the fibrous bands. Glutamine synthetase immunohistochemical stain shows an irregular blotchy/patchy pattern “maplikepattern”. While it has diffuse staining pattern in both hepatic adenomas (Beta catenin mutated) and HCC. The lesional cell show shows low proliferation index by Ki 67 immunohistochemical stain (<1%). Glypican-3 immunostain is negative. Reticulin special stain demonstrates normal reticulin pattern. It was hypothesized that FNH might be a precursor to FLC. However, there has been no proof until now to support this idea. The FNH that surrounds a small subset of FLC considers as a reaction to the tumor itself and not a precursor. [6]
Fibrolamellar carcinomas (FLCs) arise in noncirrhotic livers of older children and young adults. They compromise about 1%-9% of all HCCs according to the SEER (Surveillance, Epidemiology and End Result program) database. [1] Edmondson described FLC in 1956 [2] then term FLC was suggested in1980 by Craig et al; [3] However; it took until 2010 for the World Health Organization (WHO) to designate this clinical entity with its own WHO classification number. [4] They usually present with nonspecific signs and symptoms along with normal serum AFB levels, but in ~ 5-10%, the AFB level could reach up to 200 ng/mL. FLCs have an overall better prognosis than other primary liver tumors (e.g., HCC, intrahepatic cholangiocarcinoma). [5] The most important prognostic feature is whether the tumor is resectable or not. [6,7]
Microscopically, the background livers are non-cirrhotic in FLCs. The tumor is composed of large polygonal cells with abundant eosinophilic cytoplasm (rich in mitochondria and lysosomes), large vesiculated nuclei and prominent nucleoli. They have prominent intratumoral fibrosis, showing lamellar pattern (composed of type I, III, and V collagen). These histological findings are the definition of FLCs. In about two thirds of the cases, the fibrosis will coalesce into central scars with radiating fibrous bands. In about half of the cases, the tumor cells can have round amphiphilic cytoplasmic inclusions termed pale bodies. Hyaline bodies are also eosinophilic and tend to be smaller than the pale bodies. These bodies are not specific for FLCs but also seen in ordinary HCC. As shown in this case, intratumoral cholestasis is present in most of FLCs with canalicular bile plugs (the most common pattern). Occasional granulomas present within the normal hepatic parenchyma as well as the tumor (observed in this case). [6] It is less common to see mitosis comparing to the usual hepatocellular carcinoma [8] while vascular invasion occurs in 40–50% of FLC cases. [6,7] Some cases of FLC can show areas of gland-like formation with mucin production and they should not be called biphenotypic HCC or combined FLC and cholangiocarcinoma.
Immunohistochemical staining of FLC has some similarities to HCC, including staining positive for hepatocyte paraffin 1 (HepPar-1) but unlike most HCC, FLC stains positive for CK7 and negative for alpha fetoprotein (AFP). CD68 is often positive due to abundant tumor lysosomes. Glypican 3 is positive in only subset of cases. [5] Although there might see nneuroendocrine features; most of the cases are negative for chromogranin and synaptophysin by immunohistochemistry. [9] Honeyman et al discovered that in 100% of the FLCs (n=10 patients) aside from heterozygous 400 kb deletion on chromosome 19 (which encodes for a functional and chimeric protein) there were no other recurrent structural variations that contribute to the tumor genotype. This deletion produces an in-frame fusion of DnaJ heat shock protein family member B1 (DNAJB1) and protein kinase cAMP-activated catalytic subunit alpha (PRKACA). The absence of a second-hit mutation in the genomic background of FLC marks the DNAJB1-PRKACA fusion protein as the greatest object for diagnostic and therapeutic developments. [5, 10, 11] Recent study by Kastenhuber et al revealed that Dnajb1–Prkaca gene fusion drives tumorigenesis in mice, and that fusion to DNAJB1 drives FLC initiation more effectively than wild-type PRKACA overexpression. The requirement of the PRKACA kinase domain in tumor initiation establishes the potential utility of kinase inhibitors targeting the fusion. [12]
Hepatic adenoma is an uncommon, benign, hepatic neoplasm that typically occurs in women of childbearing age, often with a history of long-term use of oral contraceptive drugs. This is usually detected as an incidental mass lesion in a noncirrhotic liver during imaging studies. Pathologic evaluation by needle core biopsy remains the gold standard for diagnosis. Molecular studies have revealed that hepatic adenomas involve unique molecular pathways that are distinct from hepatocellular carcinoma. Based on these studies, a French collaborative group has recently proposed a molecular pathologic classification for hepatic adenomas. In addition, advances in molecular studies have led to reclassification of the ‘‘telangiectatic variant of focal nodular hyperplasia’’ as ‘‘hepatic adenoma, inflammatory subtype.’’[6]
Hepatic adenomas (except the androgen related subtype) and FNH do not have cytologic atypia or mitosis. They both usually occur in young middle-aged women. While FNH do not has malignant potential, 10% of the hepatic adenoma reported to in the literature have underwent malignant transformation. Inflammatory/ telangiectatic adenoma might be somehow a challenge to differentiate from FNH (especially on biopsy). The bile ductular proliferation in the inflammatory/telangiectatic hepatic adenoma is typically patchy and located in faux portal tracts and not fibrous bands. They typically have dilated (telangiectatic) and congested sinusoids, with large aberrant arteries in the parenchyma. They might have inflammation (but not seen in all cases). This type of adenoma is positive for serum amyloid A (which is negative in the normal liver). Some cases might also be Beta Catenin nuclear positivity; which then will be called Inflammatory/telangiectatic hepatic adenoma with Beta catenin activation. Glutamine synthetase is negative in the inflammatory/telangiectatic type, while it shows map like pattern in FNH. It could be positive in Beta catenin mutated type.
Scirrhous hepatocellular carcinoma is very rare, about 1% of the liver tumors. It is usually sub capsular, occurs in both cirrhotic (one third) and noncirrhotic (two thirds) livers. It grows as an aggregate of adjacent tumor nodules with entrapped intratumoral portal tracts. The tumor grows in atrophic cord and irregular aggregate that embed in a dense fibrous background. The fibrous areas may have lymphocytic rich inflammation (marked CD8+ predominant lymphocyte infiltrate). [14] The fibrosis can have few patterns such as thickening along the sinusoids, to pattern that is distinctly lamellar to irregular but interconnected clumps (which cause the tumor cells to grow in irregular nodular pattern). However, the best definition is that the fibrosis component makes up approximately 50% of the tumor area. For which the imaging studies are often not typical for HCC and usually the DDX from imaging studies will be as cholangiocarcinoma, metastatic carcinomas or biphenotypic HCC. [15] Tumor cells may have fatty change or clear cell change, hepatocytic inclusions can also be seen (both pale and eosinophilic bodies). The prognosis is similar to the typical HCC. Immunohistochmical stains can be helpful because FLC will be positive for CK7, CD68 and HepPar-1 whereas scirrhous carcinomas are CD68-negative and HepPar-1 negative in about 50% of the cases. CK7 is positive in all FLCS and about two thirds of scirrhous carcinomas.
Cirrhotomimetic hepatocellular carcinoma (Diffuse HCC / Cirrhotic-like HCC) grows in small nodules that closely mimic cirrhosis and in some cases. Usually seen in cirrhotic liver. Very small tumorlets can be seen inside benign cirrhotic nodules. Imaging studies and macroscopic examination may fail to show tumor. In some cases, the tumor nodules may coalesce into a larger predominant nodule, which then could be seen grossly. [16, 17] The prognosis is not known due to the rarity of this type.
References:
- National Cancer Institute D SRP. Cancer Statistics Branch Surveillance, Epidemiology, and End Results (SEER) Program. Available from: http://www.seer.cancer.gov.
- Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child. 1956;91(2):168–186.
- Craig JR, Peters RL, Edmondson HA, Omata M. Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer. 1980;46(2):372–379.
- Bosman FT, World Health Organization . WHO Classification of Tumours of the Digestive System. 4th ed. Lyon: International Agency for Research on Cancer; 2010. International Agency for Research on Cancer.
- Lafaro KJ, Pawlik TM. Fibrolamellar hepatocellular carcinoma: current clinical perspectives. J Hepatocell Carcinoma. 2015 Oct 9;2:151-7. doi: 10.2147/JHC.S75153. eCollection 2015. Review. PubMed PMID: 27508204; PubMed Central PMCID: PMC4918295.
- Torbenson M. Fibrolamellar carcinoma: 2012 update. Scientifica (Cairo). 2012;2012:743790. doi: 10.6064/2012/743790. Epub 2012 Sep 23. Review. PubMed PMID: 24278737; PubMed Central PMCID: PMC3820672.
- Stipa F, Yoon SS, Liau KH, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer. 2006;106(6):1331–1338.
- Farhi DC, Shikes RH, Murari PJ, Silverberg SG. Hepatocellular carcinoma in young people. Cancer. 1983;52(8):1516–1525
- Ward SC, Huang J, Tickoo SK, Thung SN, Ladanyi M, Klimstra DS. Fibrolamellar carcinoma of the liver exhibits immunohistochemical evidence of both hepatocyte and bile duct differentiation. Modern Pathology. 2010;23(9):1180–1190.
- Honeyman JN, Simon EP, Robine N, et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science. 2014;343(6174):1010–1014.
- Darcy DG, Chiaroni-Clarke R, Murphy JM, et al. The genomic landscape of fibrolamellar hepatocellular carcinoma: whole genome sequencing of ten patients. Oncotarget. 2015;6(2):755–770.
- DNAJB1–PRKACA fusion kinase drives FL-HCC Edward R. Kastenhuber, Gadi Lalazar, Shauna L. Houlihan, Darjus F. Tschaharganeh, Timour Baslan, Chi-Chao Chen, David Requena, Sha Tian, Benedikt Bosbach, John E. Wilkinson, Sanford M. Simon, Scott W. Lowe Proceedings of the National Academy of Sciences Dec 2017, 114 (50) 13076-13084; DOI: 10.1073/pnas.1716483114.
- Dhingra S, Fiel MI. Update on the new classification of hepatic adenomas: clinical, molecular, and pathologic characteristics. Arch Pathol Lab Med. 2014 Aug;138(8):1090-7. doi: 10.5858/arpa.2013-0183-RA. Review. PubMed PMID: 25076298.
- Kojiro M. Pathology of Hepatocellular Carcinoma. Hoboken: Wiley; 2009. pp. 1–184.
- Kim SR, Imoto S, Nakajima T, Ando K, Mita K, Fukuda K, Nishikawa R, Koma Y,Matsuoka T, Kudo M, Hayashi Y. Scirrhous hepatocellular carcinoma displaying atypical findings on imaging studies. World J Gastroenterol. 2009 May 14;15(18):2296-9. PubMed PMID: 19437576; PubMed Central PMCID: PMC2682251.
- Jakate S, Yabes A, Giusto D, Naini B, Lassman C, Yeh MM, Ferrell LD. Diffuse cirrhosis-like hepatocellular carcinoma: a clinically and radiographically undetected variant mimicking cirrhosis. Am J Surg Pathol. 2010 Jul;34(7):935-41.doi: 10.1097/PAS.0b013e3181ddf52f. PubMed PMID: 20463569.
- Han YS, Choi DL, Park JB. Cirrhotomimetic type hepatocellular carcinoma diagnosed after liver transplantation--eighteen months of follow-up: a case report. Transplant Proc. 2008 Oct;40(8):2835-6. doi: 10.1016/j.transproceed.2008.07.012. PubMed PMID: 18929876.