
Check back soon. NOFO will be announced on August 19, 2024.
Contact
Our program invites proposals seeking to overcome barrier(s) in the conduct of research as to minimize or eliminate these issue(s) in subsequent work focused on ameliorating health condition(s) that disproportionately affect our region. Research plans may lie at any point along the translational science spectrum.
-
Eligibility
Investigators from CCTS Partner Network institution(s).
-
Funding
Applicants may request up to $30,000 (direct).
-
Key Dates
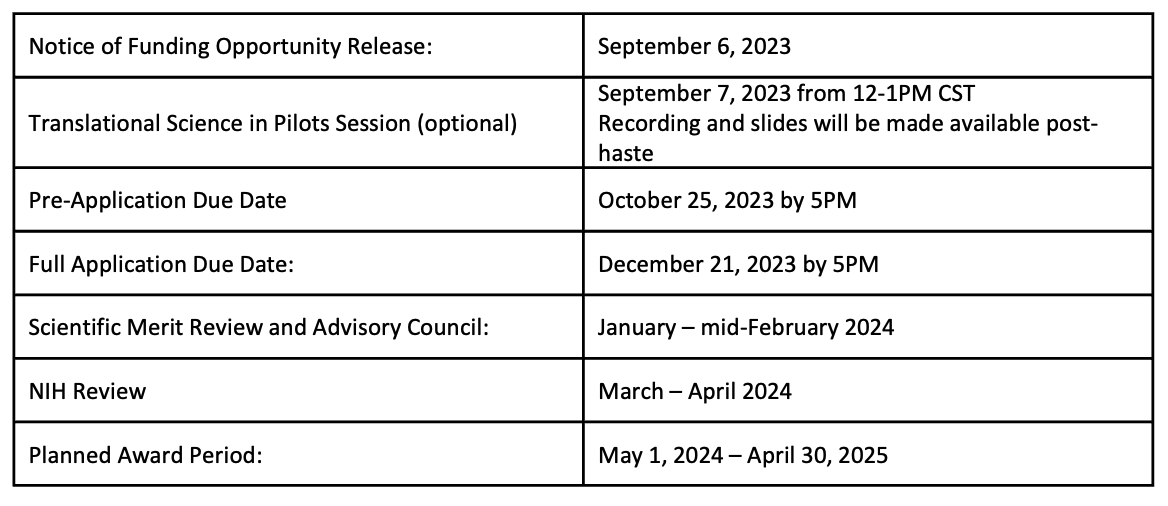
-
Translational Science in Pilots Presentation
On Sept. 7, 2023, the CCTS hosted a presentation providing a deeper understanding of translational science's role in CCTS pilot projects and how to acquire the skills needed to frame applications. Drs. Byron Lai and Sara Cooper also shared their firsthand experiences as CCTS Pilot Awardees.
View the presentation and slide deck.