-
What is translational science?
- A field of investigation focused on understanding the scientific and operational principles underlying each step of the translational process
- A key tenant of translational science is understanding common causes of inefficiency and failure in translational research projects
- Application of scientific and operational innovation and strategies to improve the efficiency and effectiveness of all research
- Examples of translational science include, but are not limited to, identifying causes and application of strategies to overcome incorrect predictions of toxicity or efficacy of new drugs, lack of data interoperability, inefficient clinical study/trial operations, ineffective clinical study/trial recruitment/retention/diversity, inefficient clinical research administrative and regulatory processes, barriers to clinical adoption and patient knowledge/engagement, gaps in public health crisis preparedness, health policy changes and dissemination
- Examples of translational science in pilots
- The Clinical Trials Transformation Initiative provides case studies, recommendations and resources
-
What are the CCTS Pilot Program timeline and activities?
- Program Overview
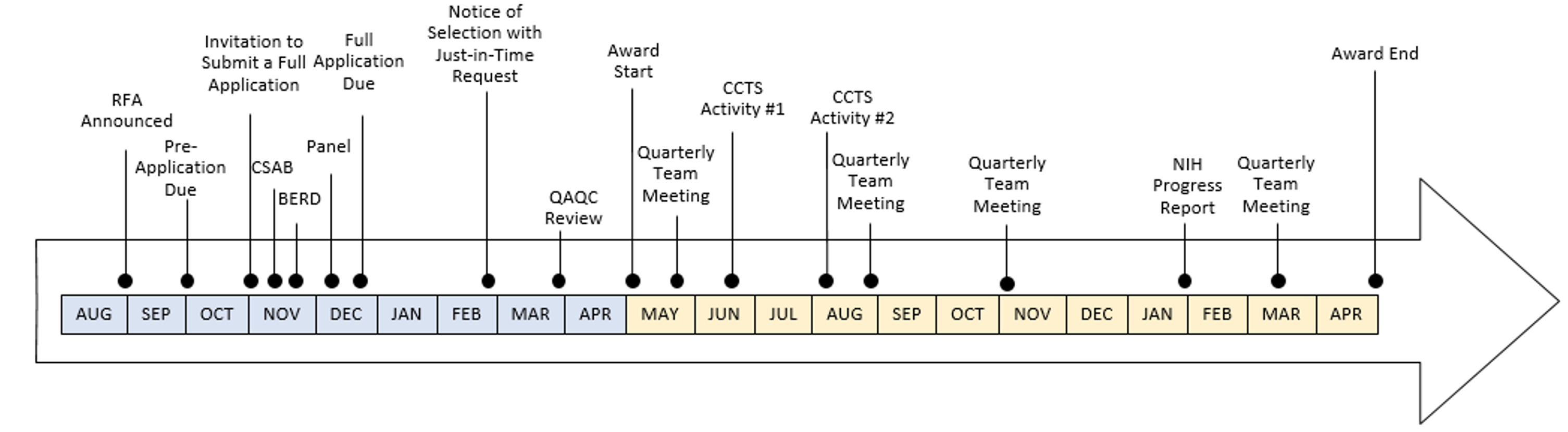
- What occurs between submitting a pre-application and full application?
- Pre-Application Due Date: Initial applications are due October 25.
- Invitation to Submit a Full Application: Invitations are supplied in early November.
- CSAB: Applications involving human subjects research, including community-engaged and/or community-based participatory research, will be discussed with the CCTS Community & Scientific Action Board. The board’s comments will be supplied back to the investigator to inform the development of the application.
- BERD: Applicants will meet with a Biostatistics, Epidemiology and Research Design team member to discuss the study design and analytical plans. Only a pre-application needs to be supplied in order to have this discussion. A BERD member will reach out to you to schedule this consult.
- Panel: Applicants will meet with a panel of peer experts to discuss their research plans. Applicants are welcome to identify panel members. The CCTS may also add members. Plan to provide your panel members with a draft, well developed or not, approximately five days BEFORE the date of the panel. A CCTS Research Commons team member will reach out to you to schedule this meeting.
- Applicants are expected to consult with any other facilities and/or cores to discuss feasibility and budget to support activities articulated in the experimental plan.
- Full Application Due: Full applications are due December 21.
- What happens when selected for the award?
- Notice of Selection with Just-in-Time Request: Applicants will be notified if they’ve been selected for award. Awards are contingent on applicants completing Just-in-Time request. Akin to the NIH Just-in-Time request, applicants provide the NIH (via the CCTS) with additional information. In the context of the CCTS Pilot, applicants are expected to furnish relevant regulatory approvals and related information, as applicable (e.g. IRB Approval, Clinicaltrials.gov registration, IACUC) and project team member recommendations. This information is then transmitted by the CCTS to the NIH for review and approval. Considering human subject and vertebrate animal research regulatory approvals can take several months to acquire, applicants should *consider submitting regulatory protocols before receiving a Notice of Selection*.
- QAQC Review: Before your Just-in-Time information is transmitted to the NIH, it undergoes a quality review. Discrepancies between the information provided and requirements for submission will be supplied to the investigator to address before the information is sent to the NIH for approval.
- Award Start: The CCTS Pilot Project Award period spans May 1 – April 30 of the following year. Delay in NCATS Prior Approval may delay the project start or award. The CCTS cannot extend awards beyond April 30 (i.e., no cost extensions are not allowable).
- What occurs during the award period?
- Quarterly Team Meetings: Quarterly team meetings are facilitated by the CCTS and include members identified by the awardee and CCTS. The first meeting occurs within weeks of the award start and occur quarterly thereafter. Awardees are expected to update their team on deliverables, such as those defined by their project timelines and enrollment plans.
- CCTS Activities: Awardees are expected to engage with CCTS events and activities several times during their award period as part of fostering the development of the translational science workforce.
- NIH Progress Report: Awardees furnish a NIH Progress Report that, if applicable, includes an enrollment update.
- What happens after the award period?
- Cite the CCTS Grant. Publications, presentations, posters, press releases or other outcomes resulting from research supported whole or in part by the CCTS are required to Cite the CCTS Grant.
- Comply with NIH Public Access Policy, evidenced by publications assigned a PMCID.
- Provide periodic metrics.
-
Are you seeking to better understand regulatory approvals and Just-in-Time requests?
- Who can help with Human Subject Research training, ethics (IRB) review and related regulatory requirements?
- CCTS Partner Institutions’ human subject research offices provide necessary training and review human subject research protocols to assure ethical conduct of research.
- UAB’s Office of the IRB and Virtual Office Hours
- Tuskegee University’s Human Participants Committee
- Auburn’s Office of Human Research
- University of Alabama at Tuscaloosa’s Institutional Review Board
- University of South Alabama’s Research Compliance and Assurance
- University of Mississippi’s Institutional Review Board
- Louisiana State University Health Science Center New Orleans’ Institutional Review Board
- Pennington Biomedical Research Campus’ Human Research Protection Program
- Tulane University’s Human Research Protection Office and IRBs
- Some Departments have Regulatory specialists as an administrative resource
- The CCTS Clinical Regulatory Support Program may be leveraged to assist investigators with regulatory questions or needs.
- CCTS Partner Institutions’ human subject research offices provide necessary training and review human subject research protocols to assure ethical conduct of research.
- What are NIH-defined clinical trials?
- The NIH’s definition of a clinical trial is a research study where one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. The NIH provides additional information and a decision tool to help determine if a study meets the definition.
- Will I need to register my study in ClinicalTrials.gov?
- If your study meets the definition of an NIH-defined clinical trial, the study must be registered in ClinicalTrials.gov and results reported accordingly.
- Note: If the study is an NIH-defined clinical trial, many institutions require investigators to submit to ClinicalTrials.gov before submitting a human subjects research protocol or before IRB approval will be granted.
- What are Investigational New Drug or Biologic (IND) and Investigational Device Exemptions (IDE) submissions?
- The Regulatory Guidance for Academic Research of Drugs and Devices (ReGARDD) website provides an excellent overview of IND and IDE submissions.
- UAB’s Office of Research provides details about IND and IDE submissions
- Who can help with Vertebrate Animal training and regulatory requirements?
- UAB’s Institutional Animal Care and Use Committee
- Tuskegee University’s Institutional Animal Care and Use Committee
- Auburn University’s Institutional Animal Care and Use Committee
- University of Alabama at Tuscaloosa’s Institutional Animal Care and Use Committee
- University of South Alabama’s Institutional Animal Care and Use Committee
- University of Mississippi’s Institutional Animal Care and Use Committee
- Louisiana State University Health Science Center New Orleans’ Institutional Animal Care and Use Committee
- Tulane University’s Institutional Animal Care and Use Committee
- Where can I learn more about this program’s Just-in-Time Request?
- Applicants receiving a Notice of Selection are asked to provide Just-in-Time information. Investigators are asked to provide relevant regulatory approval(s), related documents and programmatic information using a dynamic Just-in-Time Request form. *This example is hosted by a development instance of the live version. Users are not able to “Submit” this form. Data entered will not/cannot be saved.
- Who can help with Human Subject Research training, ethics (IRB) review and related regulatory requirements?
-
How are Pilot applications reviewed?
- Pre-Applications are assigned an Impact Score (NIH 9-point scale) corresponding to the overall scientific merit of the proposal taking into account the mission alignment, investigator qualifications and likelihood of extramural competitiveness.
- Full-Applications are scored (1-9) on aspects of significance, research team, innovation, approach and overall impact.
- An NIH-style study section reviews applications and makes recommendations on funding.
-
Additional Resources
- Tips on writing a research proposal
- Address the Top 10 Problems Reviewers Cite in Applications
- 23 Questions to Guide the Writing of a Quantitative Medical Education Research Proposal
- Top ten strategies to enhance grant-writing success
- Verify the novelty of your idea by searching publications (PubMed, dimensions.ai), awarded grants (NIH RePORTER, dimensions.ai, PIVOT) and registered trials (clinicaltrials.gov)
- Read awarded grants in the CCTS Grant Library (spoiler alert: these are NOT pilot proposals)
- Tips on writing a research proposal
Pilot Toolbox
The CCTS Pilot Program is funded by the National Center for Advancing Translational Science (NCATS), an NIH Center. The program is intended to foster the development of translational science investigators and their projects through applicable collaboration, partnership and administration activities. Questions frequently asked about this program that arise during the preparation of an application, project implementation and/or the award period are addressed below.