Managing Your Award
![]()
If your application receives a fundable score, NIH will request Just In Time (JIT) information that includes other support for key personnel, IRB (Human subjects) approvals and training, IACUC (Animal subjects) approval. JIT can be requested via eRA Commons and Grants Status Update via myUABResearch. Please contact your officer when you have completed the requested information.
Once your grant is funded OSP will request a detailed budget that corresponds with the Notice of Grant Award. At this time, you will need to make sure that all approvals are met and up-to-date.
Then you are ready to manage your award……
Managing your award is very important in the success of your research. There are certain reporting requirements and modifications that may be necessary. The most common reporting requirements and modifications are listed below. Detailed information can be found at Award Management.
Common Tasks
A Guide to UAB Information Sources for Preparing and Reviewing Other Support Pages
Federal funding agencies are giving additional scrutiny of documentation submitted in support of UAB sponsored projects. Federal sponsors are especially focused on Other Support pages. UAB is taking proactive steps to provide its research community with information and tools needed to navigate this rapidly changing environment, including development of a new UAB Other Support Template with accompanying instructions.
As an additional resource, the following is a guide to available UAB information sources that can assist in preparing and/or reviewing Other Support pages. The information sources set out here are best considered tools for reconciling information to be included in an Other Support page. While Other Support documents may not necessarily identically match the information maintained in these UAB databases, it is important to ensure consistency between information maintained by UAB and information required to be included in an Other Support page. It may be helpful to document where differences exist, why certain information in UAB sources should not be included on an Other Support page and/or, as necessary, request correction of information maintained by UAB.
A Guide to UAB Information Sources for Preparing and Reviewing Other Support Pages
Change in Effort of Key Personnel
The grantee is required to notify the GMO in writing if the PI or key personnel specifically named in the NGA will withdraw from the project entirely, be absent from the project during any continuous period of 3 months or more, or reduce time devoted to the project by 25 percent or more from the level that was approved at the time of award (for example, a proposed change from 40 percent effort to 30 percent or less effort). NIH must approve any alternate arrangement proposed by the grantee, including any replacement of the PI or key personnel named in the NGA.
The request for approval of a substitute PI/key person should include a justification for the change, the biographical sketch of the individual proposed, other sources of support, and any budget changes resulting from the proposed change. If the arrangements proposed by the grantee, including the qualifications of any proposed replacement, are not acceptable to the NIH awarding office, the grant may be suspended or terminated. If the grantee wishes to terminate the project because it cannot make suitable alternate arrangements, it must notify the GMO, in writing, of its wish to terminate, and NIH will forward closeout instructions.
The requirement to obtain NIH prior approval for a change in status pertains only to the PI and those key personnel NIH names in the NGA regardless of whether the applicant organization designates others as key personnel for its own purposes.
No Cost Extensions
First Extension
At least 30 days prior to the end date of the project period, the PI or delegate should create and submit an award modification in myUABResearch. A letter with the following information should be uploaded as supporting documentation:
- NIH grant number
- Length of extension requested (recommended: 6, 9, 12 month intervals)
- Brief scientific reason for needing additional time
This letter must be signed by the PI and the department chair. If the request is not sent until after the project period ends, the approval cannot be submitted through eRA Commons, and the NIH grants management specialist may require additional paperwork (e.g. written justification, detailed budget for unobligated balance) to process the extension.
Second and Subsequent Extensions
The PI must submit a letter to OSP at least 30 days prior to the end date of the current extension period. This letter must be signed by the PI and the department chair and contain the following information:
- NIH award number
- Length of additional time needed
- Justification for additional time
- Estimated unobligated balance available for the extension
- Detailed budget for unobligated balance
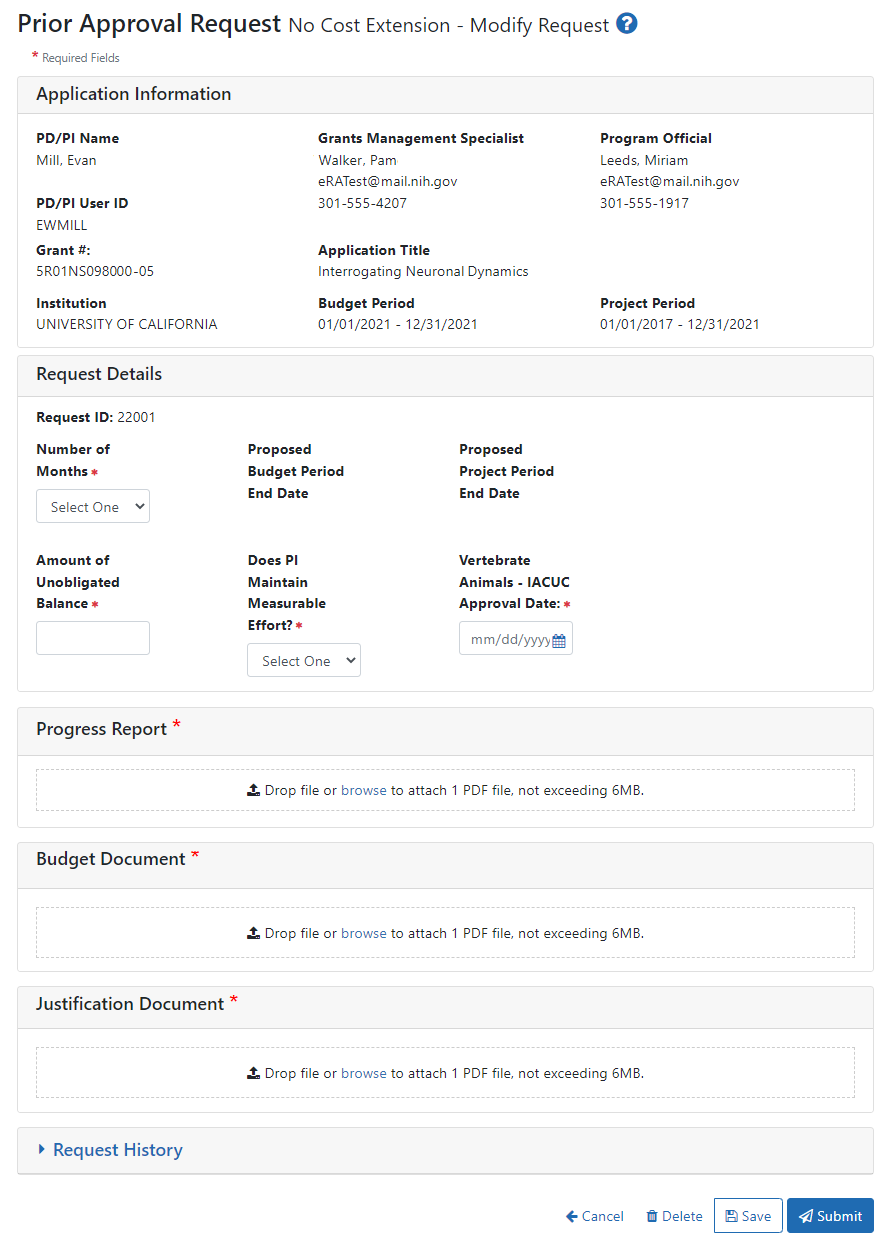
NIH No Cost Extension via Prior Approval module in eRA Commons and myUABResearch Award Modification
NIH grants that are eligible for a No Cost Extension through the eRA Commons Prior Approvals Module and not through the Status screen will need to submit the information shown below with their award modification to OSP.
All Federal sponsors No Cost Extension requests should create and submit an award modification in myUABResearch. A letter signed by the PI and Department Chair requesting a No Cost Extension should be uploaded as supporting documentation.
Please see the Required Documents Tool.
Change in PI
Procedure
When a PI will be absent from a project for three or more consecutive months or reduce effort by 25% or more, grantees must request NIH approval to appoint a permanent replacement or an interim PI who will step down when the original PI returns. To approve a new PI request, NIH issues a revised Notice of Award with the new PI.
If NIH denies a request, it gives its justifications for the rejection. Grantees can either fix the problems with their proposed new PI or nominate another investigator, but if they can't find an acceptable replacement, they must ask NIH to terminate the grant. In response, NIH issues a revised Notice of Award with a project period end date that coincides with the PI's departure date.
Principal Investigators
Request a change in PI by creating and submitting an award modification via Demographic Changes Only/Personnel Change in myUABResearch. A letter signed by the PI and Department Chair requesting a change in PI should be uploaded as supporting documentation. Include the following information:
- Reason for change.
- Date when the original PI will return, if applicable.
- Biographical sketch of the proposed new PI
- Other Support Document
- Certification of human subjects training if the proposed new PI will be working with human subjects.
- Budget changes resulting from the change in PI.
For a change of PI on a construction grant, do the following:
- Make sure the proposed new PI has the following roles:
- Highly-placed institutional official at the level of dean or equivalent.
- Responsibility and authority for research activities at the grantee organization.
- Authorization to commit institutional funds and resources.
- Your organization should submit a justification via letter or email signed by an authorized business official.
Sign the request
- Letters should include signatures of the current PI, the proposed replacement PI, and the authorized organizational official.
- Emails must reflect the concurrence of the authorized organizational official and his or her signature block, as required by policy.