 |
Gorgas Case 2019-09 |
 |
|
The Gorgas Course in Clinical Tropical Medicine 2019 spent its last week with a 4-day field trip to Iquitos, Peru on the banks of the Amazon River. Iquitos, with a population of approximately 450,000, is the largest city in the world that is reachable only by air or by river. The nearest road ends over 400 km away. The following patient was seen in the inpatient service of Regional Hospital of Loreto. We would like to thank Dr. Isela Machaca, and our Pathologists at Cayetano Heredia Hospital, Dr. Jaime Cok and Dr. Yessenia Salas for their contribution in presenting this case.
|
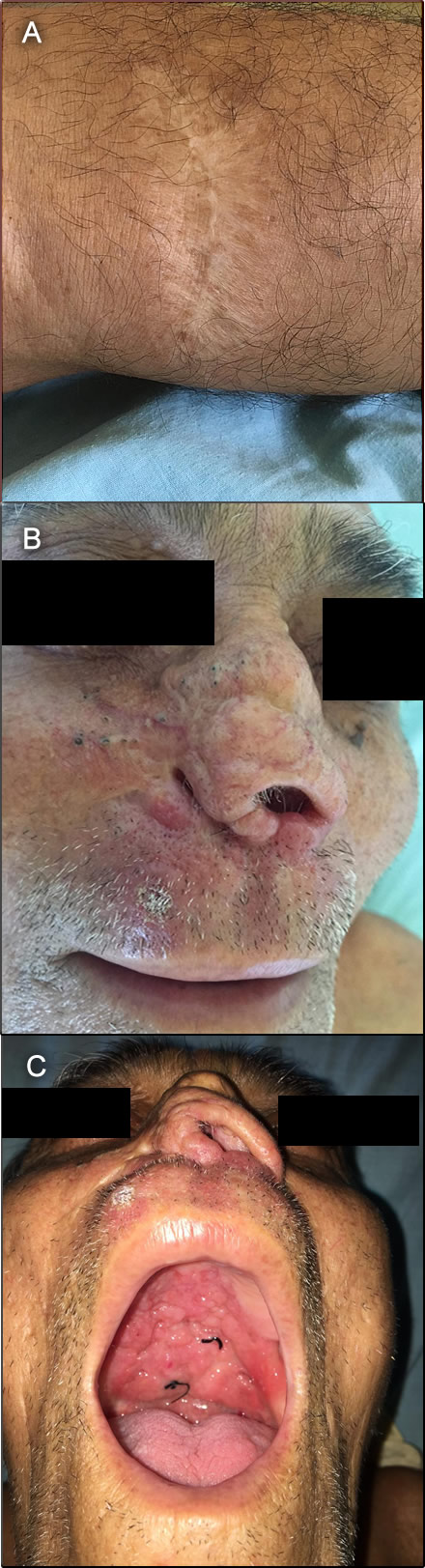 History: A 66-year-old male patient was admitted with an 8-month history of hoarse voice and odynophagia initially to solid food but also to liquids. The patient noticed an extensive lesion on the palate and significant weight loss for which decided to attend the hospital for further advice.
Epidemiology: Born and lives in Iquitos. Works as a farmer. He states that he had a painless ulcerative lesion on the left leg in 1969 while staying in Brazil, that he cured himself by applying battery acid (Image A). At that time the ulcer healed in one month with no further relapse. In 2014, he developed erythematous lesions on the nasal mucosa that were presumptively diagnosed as rhinoscleroma and treated locally with antibiotics and surgical debridement resulting in septal perforation and gross nose deformity. In addition, he states that he had two episodes of malaria in 1988.
Physical examination: Temperature 36.5C; BP: 100/60; pulse 67; respirations 20; body weight 60Kg
Gross deformity of the nose with septal perforation was found (Image B); gross granulomatous tissue infiltrating the hard and soft palate with no bleeding (Image C); absence of uvula; infiltration on the larynx including the epiglottis and vocal cords. A hypochromic lesion located on the left leg was appreciated, but no regional adenopathy. Laboratory result at admission: Hemoglobin 10.g g/dl; WBC 11,840 (0 bands, 77% neutrophils, no eos); glucose 82 mg/dl; creatinine 0.91 mg/dl.
HIV non-reactive; VDRL non-reactive; HTLV-1 non-reactive; total core positive for HVB (further studies pending). Normal chest x-ray. Stool for ova and parasites: negative UPCH Case Editors: Carlos Seas, Course Director / Carlos McFarlane, Associate Coordinator UAB Case Editors: German Henostroza, Course Director / David O. Freedman, Course Director Emeritus |
Diagnosis: Mucosal leishmaniasis
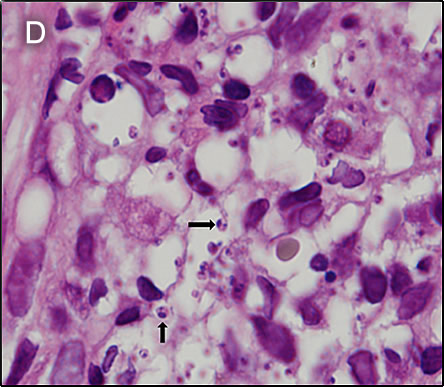
 Discussion: Biopsies were taken from the palate lesion. The pathology reported the presence of a dense inflammatory infiltrate mainly composed of histiocytes and lymphocytes. Several rounded structures were observed inside the histiocytes compatible with amastigotes, some of them disclosed a typical kynetoplast (Image D). PCR to further characterize the species is pending. L. braziliensis is the most likely Leishmania species present in the area of the jungle where he lived at the time of his initial infection. However, in South America, it is important to definitively distinguish Leishmania species that cause only cutaneous disease (e.g., L. peruviana in Perú) from the mucocutaneous species. Both typically cause one or a few initial skin lesions that are ulcerative but painless in nature and that usually spontaneously heal over time. However, with L. braziliensis (the mucocutaneous species), severe destructive recurrence may occur in the mucosal surfaces of the naso- and oropharynx from months to years after treatment or healing of the skin ulcers. L. braziliensis only occurs in the Americas and is the only Leishmania species that causes mucocutaneous disease. In this part of the world, the vector is the Lutzomyia sandfly. The major differential diagnosis in Perú of these oro-pharyngeal lesions would be paracoccidioidomycosis, carcinoma, or lymphoma. This would be an atypical presentation for TB. In Perú leishmaniasis would be by far the most common. The painless nature of the mucosal lesions despite widespread destruction of tissue, involvement of the mucosa, as well as spread to the larynx are consistent only with leishmaniasis. A search for the scar of the original cutaneous lesion, often subtle, usually on a limb, is a key part of the physical examination. Its absence should lend doubt to the impression of leishmaniasis. In general, oral lesions of paracoccidioidomycosis are painful, are frequently friable and bleed on contact, and gingival and buccal mucosa are frequently involved. Gingiva and buccal mucosa are less often involved in leishmaniasis making this case atypical. The lungs are the primary site of infection in paracoccidioidomycosis and the x-ray is generally abnormal. KOH preps of direct scrapings will be positive in up to 90% of cases of paracoccidioidomycosis with oral lesions. The presumptive diagnosis of rhinoscleroma in this patient is doubtful in light of the clinical evolution. Distinguishing L. braziliensis from L. peruviana had for many years involved laborious culture techniques followed by electrophoretic isoenzyme analysis. Investigators at our Institute have now published PCR assays using both tissue as well as less invasive specimens [PLoS One. 2011;6(10):e26395; Clin Infect Dis. 2010 Jan 1;50(1):e1-6] for distinguishing the 2 species from cutaneous and mucosal lesions. Early identification of the species that causes the initial cutaneous infection would greatly help to prevent mucocutaneous leishmaniasis because it would allow more aggressive treatment and follow-up. In Perú L. peruviana (cutaneous disease only) occurs only in the high Andes; L braziliensis occurs only in the jungle and in the Amazon. Recommended standard therapy for limited mucocutaneous disease that does not extend beyond the uvula is often 28 days of a pentavalent antimonial (sodium stibogluconate 20mg/kg/d). In our Institute, we usually begin with amphotericin B in cases of mucosal involvement when extension is beyond the uvula, as in this case. Patients are given pre- and post-treatment hydration and supplemental potassium with daily doses given in an outpatient infusion therapy setting. Liposomal amphotericin may be used in resource-rich settings, but based on limited trials it is not better in terms of efficacy even if less toxic and more convenient. Oral miltefosine is also an alternative, but failures, toxicity and the need for larger trials limits its use. On ENT examination the patient was found to have laryngeal involvement defining it as a severe case [see Gorgas Case 2010-01]. Response is expected to be slow, with the expectation of an 80% cure rate at the end of 6-week course (25 mg/kg total dose) of Amphotericin B 6 days per week as an outpatient. For severe refractory cases, the duration of amphotericin B is extended; therapy needs to be individualized. Parasite loading may be a biomarker of treatment success in mucosal leishmaniasis [Am J Trop Med Hyg 2016;94:107]. Clearly, new treatment alternatives are needed [Rev Soc Bras Med Trop 2018;51:120]. |